Stimuli-responsive polymer-dasatinib prodrug to reprogram cancer-associated fibroblasts for boosted immunotherapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
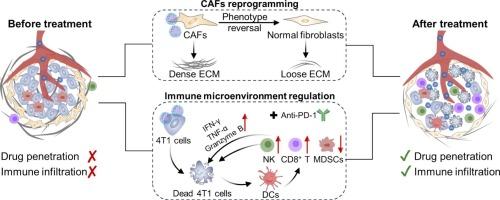
The barriers from cancer-associated fibroblasts (CAFs) have diminished the clinical efficacy of immunotherapy for triple-negative breast cancer (TNBC). The obstacles from CAFs often result in poor drug penetration, constrained cytotoxic T lymphocyte infiltration, and an immunosuppressive microenvironment. Herein, chondroitin sulfate (CS) was engineered to conjugate dasatinib (DAS), a tyrosine kinase inhibitor, via the cathepsin B (CTSB)-responsive GFLG linker to produce CS-GFLG-DAS (CGD), which could be employed to reverse the CAF phenotype and regulate the biosynthesis of extracellular matrix (ECM), thus enhancing the efficacy of immune checkpoint blockade (ICB) therapy. Upon reaching the tumor site, DAS released from CGD in response to overexpressed CTSB in the tumor microenvironment could transform CAFs into a quiescent state instead of killing them to prevent CAFs from producing abundant ECM, thereby promoting deep penetration of CGD to effectively kill tumor cells. In addition, ECM remodeling facilitated tumor infiltration of cytotoxic T lymphocytes, synergistically enhancing the anti-PD-1 efficacy in the 4T1 tumor-bearing mice. In summary, this prodrug enhanced deep drug penetration and therapeutic sensitivity of anti-PD-1 by regulating CAFs, providing new insights into optimizing immunotherapy in treating fibrotic tumors via nanomedicine.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


