Heterostructure-Derived Heterovalent Fe(OH)2/Fe Pair Sites: Tuning Adsorption of Intermediates and Enhancing Utilization of Atomic *H for Efficient Nitrate Reduction to Ammonia
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
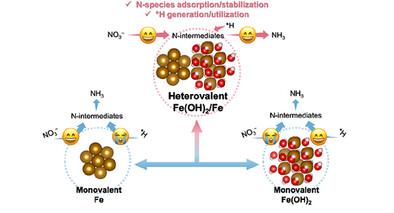
Electrocatalytic nitrate reduction (NO3RR) to valued ammonia is an ideal supplementary route to the Haber–Bosch method and a strategy for the removal and utilization of nitrate pollutants. However, due to the fact that NO3RR goes through a complicated multi-electron/proton transfer, catalysts with monovalent metal sites are difficult to tackle multitasking that it involves, leading to unsatisfactory nitrate conversion efficiency and ammonia selectivity. Herein, heterovalent Fe(OH)2/Fe pair sites supported onto carbon nanotubes (Fe(OH)2/Fe@CNTs) are presented via electrochemical reconstruction of CNTs-supporting FeS/Fe2C heterostructure. Fe(OH)2/Fe@CNTs exhibits a high NH3 yield rate of 0.67 mmol h−1 cm−2 with a FE of 95.1% at −0.4 V versus RHE, which is mainly attributed to the regulated electronic structure and cooperation of heterovalent iron pair sites. Meanwhile, the adsorption of nitrogen-containing species is adjusted and the utilization of *H is enhanced. Moreover, a balanced content of Fe(OH)2 and Fe creates “buffering effect” to maintain its activity and stability. Theoretical calculations combined with in situ FTIR and in situ Raman spectra reveal a novel multiple reaction pathway on heterovalent Fe(OH)2/Fe pair sites, entirely different from a single pathway on monovalent Fe or Fe(OH)2. Clearly, this study offers a creative strategy for the design of advanced catalysts with multivalent metal sites.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


