Hydrated Electrons Trigger the Breakdown of Recalcitrant Cyanuric Acid in Wastewater
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
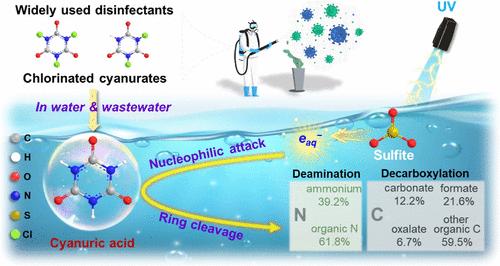
Cyanuric acid (CA), a triazine-ring compound commonly used as a stabilizer for free chlorine to enhance disinfection, often persists in wastewater for the production of chlorinated cyanurates (Cl-CAs), posing challenges for treatment. This study demonstrates that conventional advanced oxidation processes (UV/H2O2 and UV/peroxydisulfate) are ineffective in degrading CA, while the UV/sulfite system successfully achieves its breakdown. Hydrated electrons (eaq–) were identified as the primary reactive species responsible for cleaving the stable triazine ring, with minimal contributions from SO3•– and H•. The pH value influences both the activity of eaq– and the degradability of CA by altering its structure; lower pH increases the electron-deficient regions in dihydrogen CA, enhancing its susceptibility to nucleophilic attack by eaq–. The high concentrations of Cl– can inhibit CA removal, likely due to the formation of reactive chlorine species that react with sulfite and suppress eaq– production. Effective CA degradation was also demonstrated in real wastewater, highlighting the UV/sulfite system as a sustainable solution for water treatment. These findings offer valuable insights into CA transformation and present effective approaches for eliminating emerging contaminants in the context of the extensive use of disinfectants.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


