Enantioselective [2π + 2σ] cycloaddition of bicyclobutanes with imines: An efficient approach to chiral 2-aza-bicyclo[2.1.1]hexanes
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
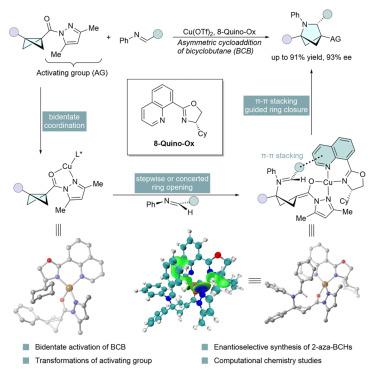
The efficient construction of bicyclo[2.1.1]hexanes (BCHs) has gained significant attention due to their potential use as bioisostere of arenes in drugs. While the synthesis of racemic BCHs has been extensively explored, strategy enabling the enantioselective assembly of such motifs remains rare. Herein, we present an efficient approach to chiral 2-azabicyclo[2.1.1]hexanes through a copper-catalyzed asymmetric [2π + 2σ] cycloaddition of bicyclobutanes with imines. With the aid of a bifunctional chiral quinolinyl oxazoline ligand, we achieved high yields and excellent ee. Density functional theory (DFT) calculations and independent gradient model based on the Hirschfeld partition (IGMH) analysis showed the π-π stacking interactions between the quinoline ring of the ligand and the phenyl ring of imine are the key to increasing both the reactivity and enantioselectivity of the reaction.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


