Hierarchical Self-Assembly of J-Aggregated 1,2-Bis(2-(benzyloxy)benzylidene) Hydrazine@2β-Cyclodextrin into Left-Handed Superhelix and Its External Stimuli-Responsive Unwinding
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
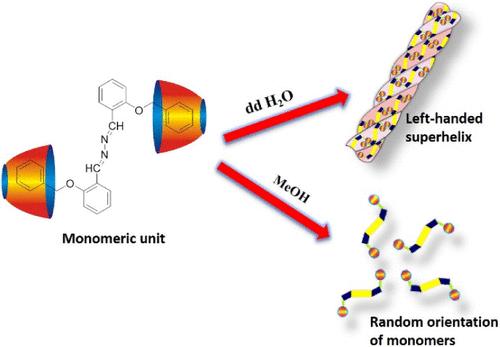
Induction of chirality to nanosized superstructures from hierarchical self-assembly of achiral monomeric units is an important area to understand the natural chiral amplification and evolution of life processes. We report herein that the complexation of salicylaldehyde azine, 1,2-bis(2-(benzyloxy)benzylidene)hydrazine (BSAZ), with β-cyclodextrin (β-CD) in aqueous solution results in the formation of a slipped J-aggregate (θ < 54.7°) that aggregates further into a left-handed superhelix through sterical constraints triggered by the hydrophobic effect. The structure of the monomeric BSAZ@2β-CD was elucidated by ultraviolet–visible (UV–vis), Fourier transform infrared spectroscopy (FT-IR), mass, powder X-ray diffraction (PXRD), and 1H, 13C, and 13C CP/MAS nuclear magnetic resonance (NMR) spectroscopy. The size, shape, and morphology of the self-aggregated hierarchy were evidenced by dynamic light scattering (DLS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) studies. The system showed excellent aggregation induced circular dichroism (AICD) with a negative Cotton effect and a high fluorescence quantum yield of 0.33 at 620 nm in a poor solvent, water, because of the formation of a higher order excimer (N ≈ 46). The helical superstructure showed responsiveness under 254 nm UV light irradiation. Light irradiation slowly unwinds the supercoiled structure into a single strand, as was visualized by a SEM image taken after 15 min of continuous light irradiation. The excellent solvatochromic effect and the control over the formed hierarchical morphology show how a supramolecular approach tailored by noncovalent interactions can develop chiral superstructures from completely achiral molecular building blocks that would have a considerable practical value in chiroptics, templates, and chiral sensing.
j聚集的1,2-二(2-(苯氧基)苄基)Hydrazine@2β-环糊精左旋超螺旋的层次自组装及其外部刺激响应解绕
从非手性单体的层次自组装中诱导手性到纳米级超结构是理解自然手性扩增和进化过程的重要领域。本文报道了水杨醛嗪1,2-双(2-(苄基氧基)苄基)肼(BSAZ)与β-环糊精(β-CD)在水溶液中络合形成滑动j聚集体(θ <;54.7°),通过疏水效应引发的空间约束进一步聚集成左旋超螺旋。通过紫外-可见(UV-vis)、傅里叶变换红外光谱(FT-IR)、质量、粉末x射线衍射(PXRD)、1H、13C、13C CP/MAS核磁共振(NMR)等手段对BSAZ@2β-CD单体的结构进行了表征。动态光散射(DLS)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)研究证实了自聚集层次结构的大小、形状和形态。该体系具有良好的聚集诱导圆二色性(AICD)和负棉花效应,在较差的溶剂水中,由于形成了高阶准分子(N≈46),在620 nm处荧光量子产率高达0.33。螺旋上部结构在254 nm紫外光照射下表现出响应性。光照射缓慢地将超螺旋结构解绕成单链,如连续光照射15分钟后拍摄的扫描电镜图像所示。优异的溶剂化变色效应和对形成的分层形态的控制表明,通过非共价相互作用定制的超分子方法如何从完全非手性分子构建块开发手性超结构,这将在手性学、模板和手性传感方面具有相当大的实用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


