Automerization of an Enediyne via a Symmetrical p-Benzyne Diradical Intermediate
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
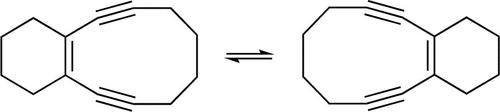
Automerization is defined as a rearrangement reaction that yields a degenerate form of the starting material. We now report that cyclohexeno[3,4]cyclodeca-1,5-diyne-3-ene rearranges to its automer, via a D2h-symmetric p-benzyne intermediate. The NMR evidence is that when the enediyne is heated to 75 °C in DMSO with either LiI or NaNO3 as possible nucleophile, the enediyne is consumed only in the presence of LiI, whereas it remains “unchanged” in the presence of NaNO3.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


