A kinetic and mechanistic study of copper-based catalysts in the ARGET-ATRP of multifunctional natural molecules: the case of methacrylated eugenol
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
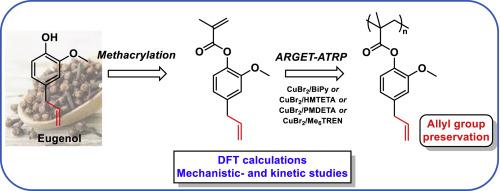
Herein we report on a kinetic study of Cu-based catalysts employed in the ARGET-ATRP of the methacrylic derivative of eugenol, namely eugenyl methacrylate (EuMA). Polymerizations were carried out in solution in presence of catalytic systems formed in situ by CuBr2 and nitrogen-ligands such as BiPy, PMDETA, HMTETA and Me6TREN. The formation of insoluble polymers, due to secondary reactions responsible of cross-linking, was observed with CuBr2/BiPy and CuBr2/HMTETA systems; Me6TREN- and PMDETA-based catalyst proved, instead, to be capable of generating linear polymers. For the latter, first order kinetics occurred for monomer conversion up to ca 50%, whilst higher conversion led to deviations from the linear trend. This suggested the direct involvement of the allyl group in the termination reactions, which was convincedly demonstrated by comparing kinetic results for EuMA and the corresponding di-hydrogenated monomer (DEuMA). At EuMA conversion above 50%, the side reactions lead to inactivation of the PMDETA-based catalytic system via reducing agent consumption rather than to the formation of insoluble/crosslinked polymers.Electronic structure calculations provided the energy profile for all possible side reactions. Among these, the radical chain transfer to the allyl group through hydrogen abstraction, as well as the attack of the propagating methacrylic radical to the allyl group, contributed to rationalizing the experimental behavior of the three copper-catalyst systems employed in this work.This study demonstrates that modulating the kinetic of polymerization by properly selecting ligands and reaction temperatures represents a useful strategy towards the reduction of undesired secondary reactions of molecules with sensitive functional groups such as bio-derived phenols; moreover, such preserved functional groups would serve as possible post-functionalization sites (i.e. epoxidation) allowing for the preparation of new materials with tailored properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


