Towards Stable Metal–I2 Battery: Design of Iodine–Containing Functional Groups for Enhanced Halogen Bond
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
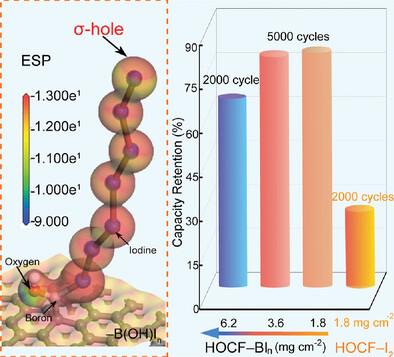
The redox chemistries of iodine have attracted tremendous attention for charge storage owing to their high theoretical specific capacity and natural abundance. However, the practical capacity and cycle life are greatly limited by the active mass loss originating from the dissolved iodine species in either non-aqueous or aqueous batteries. Despite intensive progress in physical and physicochemical confinements of iodine species (I2/I3−/I−), less attention has been paid to confining iodine species beyond the host–iodine interface, inhibiting further development of iodine cathodes with high I2 contents. Here a halogen bond (XB)– enhanced design concept is proposed between I2 molecules to achieve stable cycling performances, as exemplified by the Na–I2 battery. The enhanced XB is derived from the incorporation of –B(OH)I3 groups in highly integrated porous carbon/I2 cathode (HOCF–BIn), which can generate extended interactions between –B(OH)I3 and following I2 molecules. Due to the strong intermolecular force between I2 molecules, the HOCF–BIn cathodes exhibit substantially strengthened I2/I3−/I− confinement, enabling outstanding cycling stability at I2 loading ranging from 1.8 to 6.2 mg cm−2. This findings demonstrate a functional group to manipulate XB chemistry within I2 molecules and polyiodides for stable and low-cost metal–iodine batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


