Coordinated differentiation of human intestinal organoids with functional enteric neurons and vasculature
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Human intestinal organoids (HIOs) derived from human pluripotent stem cells co-differentiate both epithelial and mesenchymal lineages in vitro but lack important cell types such as neurons, endothelial cells, and smooth muscle, which limits translational potential. Here, we demonstrate that the intestinal stem cell niche factor, EPIREGULIN (EREG), enhances HIO differentiation with epithelium, mesenchyme, enteric neuroglial populations, endothelial cells, and organized smooth muscle in a single differentiation, without the need for co-culture. When transplanted into a murine host, HIOs mature and demonstrate enteric nervous system function, undergoing peristaltic-like contractions indicative of a functional neuromuscular unit. HIOs also form functional vasculature, demonstrated in vitro using microfluidic devices and in vivo following transplantation, where HIO endothelial cells anastomose with host vasculature. These complex HIOs represent a transformative tool for translational research in the human gut and can be used to interrogate complex diseases as well as for testing therapeutic interventions with high fidelity to human pathophysiology.
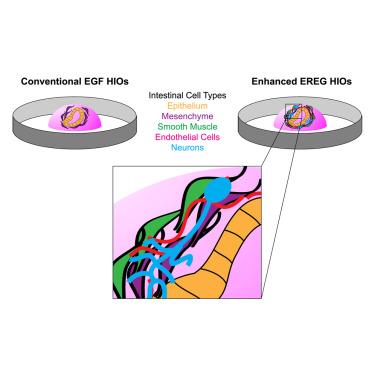
具有功能性肠神经元和血管的人肠道类器官的协调分化
来源于人多能干细胞的人类肠道类器官(HIOs)在体外可分化上皮细胞和间充质细胞谱系,但缺乏重要的细胞类型,如神经元、内皮细胞和平滑肌,这限制了转化潜力。在这里,我们证明肠干细胞生态位因子EPIREGULIN (EREG)在一次分化中增强了HIO与上皮、间质、肠神经胶质群体、内皮细胞和有组织平滑肌的分化,而不需要共培养。当移植到小鼠宿主体内时,HIOs成熟并表现出肠神经系统功能,经历蠕动样收缩,表明神经肌肉单元具有功能。在体外使用微流体装置和体内移植后,HIO内皮细胞与宿主血管吻合,证明HIO也能形成功能性脉管系统。这些复杂的hio代表了人类肠道转化研究的变革性工具,可用于询问复杂疾病以及测试具有高保真度的人类病理生理学的治疗干预措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


