Pt/α-MoC Catalyst Boosting pH-Universal Hydrogen Evolution Reaction at High Current Densities
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
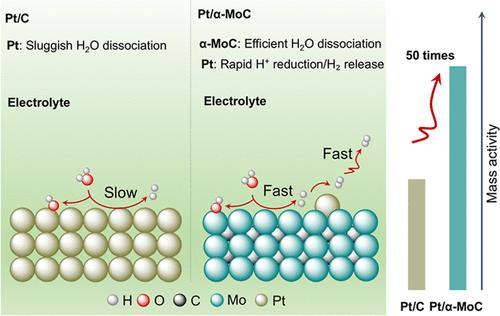
Constructing subnanometric electrocatalysts is an efficient method to synergistically accelerate H2O dissociation and H+ reduction for pH-universal hydrogen evolution reaction (HER) for industrial water electrolysis to produce green hydrogen. Here, we construct a subnanometric Pt/α-MoC catalyst, where the α-MoC component can dissociate water effectively, with the rapid proton release kinetics of Pt species on Pt/α-MoC to obtain a good HER performance at high current densities in all-pH electrolytes. Quasi-in situ X-ray photoelectron spectroscopy analyses and density functional theory calculations confirm the highly efficient water dissociation capability of α-MoC and the thermodynamically favorable desorption process of hydrolytically dissociated protons on Pt sites at the high current density. Consequently, Pt/α-MoC requires only a low overpotential of 125 mV to achieve a current density of 1000 mA cm–2. Moreover, a Pt/α-MoC-based proton exchange membrane water electrolysis device exhibits a low cell voltage (1.65 V) and promising stability over 300 h with no performance degradation at an industrial-level current density of 1 A cm–2. Notably, even at a current of 100 A, the cell voltage remains low at 2.15 V, demonstrating Pt/α-MoC’s promising potential as a scalable alternative for industrial hydrogen production. These findings elucidate the synergistic mechanism of α-MoC and atomically dispersed Pt in promoting efficient HER, offering valuable guidance for the design of electrocatalysts in high current density hydrogen.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


