Biotin Receptor-Targeting PtIV Oxygen Carrying Prodrug Amphiphile for Alleviating Tumor Hypoxia Induced Immune Chemotherapy Suppression
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
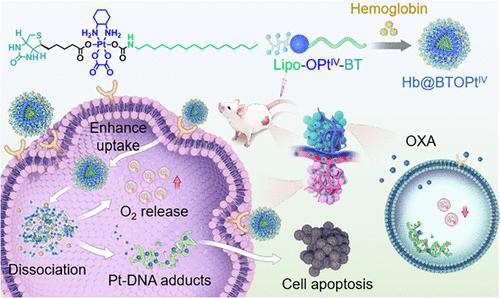
Platinum (Pt)-based chemotherapeutic agents, known for their potent cytotoxicity, are extensively used in clinical oncology. However, their therapeutic efficacy is severely limited by a variety of factors, particularly the hypoxic tumor microenvironment (TME), which not only impedes effective drug delivery but also triggers immune suppression, further diminishing the antitumor effects of Pt drugs. In response to these challenges, we have developed a biotin receptor (BR)-targeting oxaliplatin (OXA)-based PtIV prodrug, named Lipo-OPtIV-BT, which could encapsulate hemoglobin (Hb) as an oxygen carrier, forming PtIV-loaded lipid nanoparticles (Hb@BTOPtIV). The design of the Hb@BTOPtIV aims to address the dual issues of poor drug delivery and immune suppression by effectively increasing local oxygen tension in the TME. Notably, our findings demonstrate that the cytotoxic effects of the BR-targeting PtIV prodrug and increased oxygen levels synergistically reverse the tumor immune microenvironment, leading to improved antitumor efficacy. We observed that Hb@BTOPtIV significantly improved the biodistribution of the drug, enabling it to preferentially accumulate in tumor regions. Importantly, the enhanced oxygenation within the TME also plays a critical role in reshaping the immune landscape of the tumor, promoting a more favorable immune environment for effective chemotherapy. This reversal of immune suppression is evidenced by increased infiltration of cytotoxic T cells and reduced levels of regulatory T cells (Tregs) within the tumor. These findings highlight the promising potential of using BR-targeting lipid PtIV prodrug amphiphiles to improve drug accumulation at tumor sites and counteract immunosuppression induced by tumor hypoxia.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


