Preparation, separation and storage of N-monofluoromethyl amides and carbamates
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
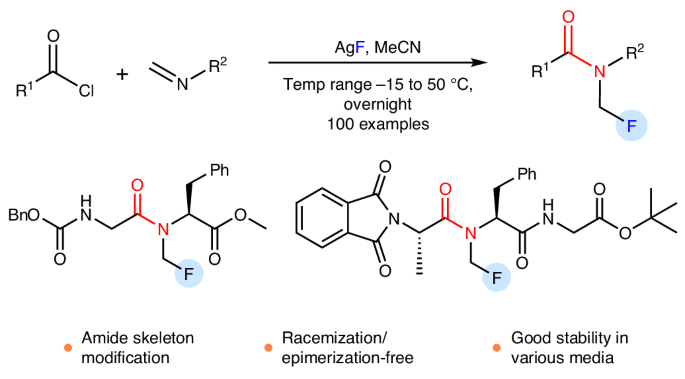
N-monofluoromethyl (N-CH2F) amides, combining amide and monofluoromethyl motifs, represent a practical modification of the amide bond that can mimic N-CH3 amides. Despite the potential value in transforming peptides and peptidomimetics with N-CH2F, the very existence of this structure has been controversial. Here we report the preparation of N-CH2F amides and carbamates via simple and robust chemical methods. The syntheses of N-CH2F amides were achieved via successive acylation and fluorination of imines and directly used in the modification of drugs, peptides and heteroaryl amides without racemization or epimerization. The use of triethylamine is the key to the separation of N-CH2F amides. The stability of nine structurally diverse N-CH2F amides was tested in eight different media, showing that most compounds remained 60–100% intact for 24 h. The monofluoromethyl (CH2F) motif is valuable as it can mimic CH3 and CH2OH motifs frequently found in bioactive molecules, but the synthesis of N-CH2F amides is challenging. Now the synthesis of numerous N-CH2F amides has been achieved via successive acylation and fluorination of imines, enriching pathways for N-methylation of biomolecules.

n -单氟甲基酰胺和氨基甲酸酯的制备、分离和储存
n -单氟甲基(N-CH2F)酰胺结合了酰胺和单氟甲基基序,代表了酰胺键的实际修饰,可以模拟N-CH3酰胺。尽管N-CH2F在转化多肽和拟肽物方面具有潜在价值,但这种结构的存在一直存在争议。在这里,我们报告了N-CH2F酰胺和氨基甲酸酯的制备通过简单和稳健的化学方法。N-CH2F酰胺的合成是通过亚胺的连续酰化和氟化实现的,并直接用于药物、肽和杂芳酰胺的修饰,而不需要外消旋化或外映异构化。三乙胺的使用是分离N-CH2F酰胺的关键。9种结构各异的N-CH2F酰胺在8种不同介质中的稳定性测试表明,大多数化合物在24 h内保持60-100%的完整性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


