Continuous flow synthesis of cyclobutenes via lithium ynolates†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
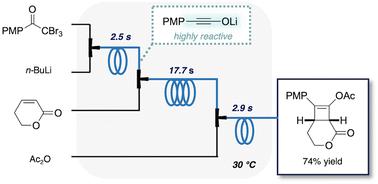
Batch reactions that involve the generation of highly reactive species require a cryogenic temperature, complicated manipulations by chemists, and higher amounts of reagents, resulting in energy wastage and high costs. In this study, we developed a continuous flow synthesis of functionalised cyclobutenes, where the first step was the flash generation of short-lived lithium ynolates. Lithium ynolates were generated by the reaction of α,α,α-tribromomethyl ketones and n-butyllithium at 30 °C in 2.5 s and transferred to the next reactor before decomposition. The optimal reaction time (2.5 s) and temperature (30 °C) were determined via in-line Raman spectroscopy. The one-flow process involved three steps: the generation of lithium ynolates, the [2 + 2] cycloaddition reaction with α,β-unsaturated esters, and acetylation of the resulting unstable lithium enolates. These reactions were mediated by several reactive chemical species such as lithium ynolates, ketenes, and lithium enolates. Our green, flash flow approach to generating ynolate anions does not require cryogenic conditions and is highly reproducible and scalable, making it suitable for practical applications.
环丁烯酸锂连续流合成研究
间歇式反应涉及高活性物质的生成,需要低温、化学家复杂的操作和大量的试剂,导致能源浪费和高成本。在本研究中,我们开发了一种连续流动合成功能化环丁烯的方法,其中第一步是闪制短寿命的烯酸锂。α、α、α-三溴甲基酮与正丁基锂在30℃下反应2.5 s生成醇化锂,并转移至下一反应器分解。通过拉曼光谱法确定了最佳反应时间(2.5 s)和温度(30℃)。这一流程包括三个步骤:醇化锂的生成,与α、β-不饱和酯的[2 + 2]环加成反应,以及不稳定的烯醇化锂的乙酰化反应。这些反应是由几种活性化学物质介导的,如烯醇酸锂、烯酮酸锂和烯醇酸锂。我们的绿色闪流方法产生壬酯阴离子不需要低温条件,具有高度可重复性和可扩展性,适用于实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


