Mechanism of anthracite dust suppression by surfactants with different alkyl chain lengths: Thermodynamic micelle morphology, adsorption probability, and interfacial forces
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
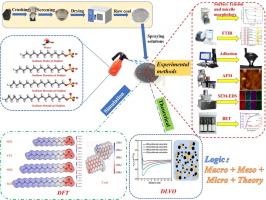
Coal dust explosions and pollution cause serious problems in the front line of mines; these issues can be effectively resolved by wetting the coal dust. In this study, the effect of the alkyl chain length of surfactants on their adsorption on anthracite surfaces and the microscopic mechanism underlying this impact was evaluated. The differences in the critical micelle concentration and micelle morphology of surfactants with varying alkyl chains in solution systems were evaluated through surface tension measurement and thermodynamic theory. In addition, the differences in the functional groups, surface energy, adhesion, roughness, pore size, and elemental distribution of coal samples modified using surfactants with various numbers of methylene groups in their alkyl chains were characterized by testing raw and adsorbed coal samples. Based on this, the mechanism underlying the effect of the alkyl chains on adsorption was elucidated from a mesoscopic perspective and the enhancement of the amount and strength of the adsorption of the surfactant on anthracite surfaces upon the shortening of the alkyl chain was explained. The high and low distributions of energies on various surfactants and coal molecules were calculated using the density functional theory, and the possible adsorption configurations were predicted. The simulation results showed that the adsorption energy of the short-chain surfactants on the anthracite surface was higher, the hydrogen bonds formed were shorter, and the charge transfer was faster, with a tendency prevailing for a stepwise increase in the alkyl chain length. Finally, the interaction mechanism between the surfactant and anthracite particles in the aqueous phase was calculated and analyzed using the classical DLVO theory. The results showed that the surfactants with shorter alkyl chains were easily dispersed with coal dust particles in water, which was favorable for collision adsorption. In addition, research directions toward the inhibition of coal dust by surfactants were discussed on the basis of the results of this study.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


