Adsorption-Induced Deformation in Microporous Kerogen by Hydrogen and Methane: Implications for Underground Hydrogen Storage
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
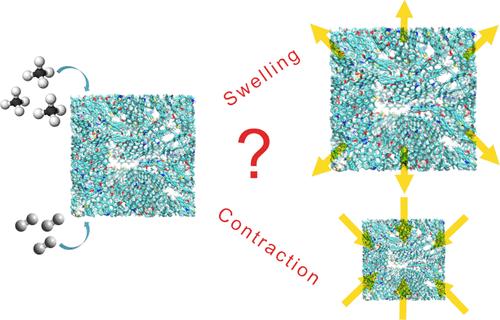
Accurately assessing the adsorption and diffusion behaviors of H2, CH4, and their mixtures are essential for estimating underground hydrogen storage (UHS). This understanding is critical for the safe and efficient storage of H2 in depleted shale gas reservoirs. Although H2 adsorption in kerogen has been extensively studied, adsorption-induced swelling remains unexplored in UHS. In this study, we investigate adsorption mechanisms using Lagrangian and Eulerian approaches and analyze diffusion in kerogen through molecular simulations. Our results reveal that in the presence of cushion gases like CH4, which exhibit stronger adsorption than H2, neglecting kerogen deformation can lead to an underestimation of storage capacity by approximately 40%. Furthermore, increasing pressure makes H2 adsorption behavior deviate from the consistent swelling trend that is observed with CH4, with kerogen either swelling or contracting depending on the pore size. Simulations also predict that H2 self-diffusion coefficient in porous kerogen is 1 order of magnitude higher than CH4. These findings highlight the importance of incorporating kerogen flexibility into the modeling of UHS involving multiple gas species to improve the accuracy and safety of H2 storage operations in shale reservoirs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


