New insights into bioactive Ga(III) hydroxyquinolinate complexes from UV-vis, fluorescence and multinuclear high-field NMR studies
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
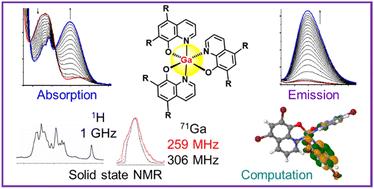
There is current interest in the anticancer and antimicrobial activities of Ga(III) tris-hydroxyquinolinate complexes, and hence their solution and solid-state chemistry. Here, we have studied the formation, stability and structure of a novel tris-5,7-dibromo-8-hydroxyquinolinate Ga(III) complex [Ga(Br2-HQ)3]. Reactions of 5,7-dibromo-8-hydroxyquinoline with Ga(NO)3 in DMSO were followed using electronic absorption and emission spectroscopy, and revealed the slow but concerted coordination of three chelated ligands, with ligand deprotonation being the apparent rate-limiting step, facilitated by basic Ga(III) hydroxido species. The emissive excited state of [Ga(Br2-HQ)3] in DMSO had a short half-life of 1.2 ns, and the fluorescence (550 nm, λex = 400 nm) was characterized by TDDFT calculations as arising from a ligand-centred singlet S1 state. We compared the structures of [Ga(Br2-HQ)3] and the clinical tris-hydroxyquinolinate complex [Ga(HQ)3] using high-field magic-angle-spinning solid-state 1D and 2D 850 MHz and 1 GHz 1H, 13C and 71Ga NMR spectroscopy. The similarity of their coordination spheres was confirmed by their 71Ga chemical shifts of 101 and 98 ppm, respectively, and quadrupolar coupling constants of 9.265 MHz and 9.282 MHz. 1H-1H 2D NOESY experiments revealed second coordination sphere interactions between an acetic acid solvent molecule and the bound hydroxyquinolinate ligands of [Ga(HQ)3]·0.5CH3CO2H. This finding suggests that carboxylic acids could play a role in modifying the formulation properties of this drug for clinical use.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


