A novel bimetallic UiO-66 loofah sponge adsorbent prepared in situ for the efficient removal of methyl orange and Cr(VI): Batch experiments and DFT calculations
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The detrimental effects of azo dyes and heavy metal ions on human ecosystems remain an ongoing issue, and so the development of methods for their removal is a growing area of research. In this study, a Ce-UiO-66-NH2@LS composite (LS = loofah sponge) with a multisite adsorption function was prepared by an in situ growth method. This combination of an LS carrier with a metal–organic framework (MOF) was found to effective in the removal of methyl orange (MO) and hexavalent chromium (Cr(VI)). At a low pH, the maximum adsorption amounts of MO and Cr(VI) were 930.58 and 282.77 mg/g, respectively, with 96.70 and 94.90 % removal being achieved under these conditions. In the presence of multiple anions, Ce-UiO-66-NH2@LS demonstrated a strong anti-ion interference ability for the adsorption of MO and Cr(VI), and maintained a high adsorption performance in a mixed MO–Cr(VI) system. Four adsorption isotherms and kinetic models were used to evaluate the adsorption process, and it was deduced that chemisorption was the dominant process. The adsorption mechanism was further analyzed by X-ray photoelectron spectroscopy and using the density functional theory approach. Consequently, electronic interactions and coordination effects were found to be the main mechanisms of adsorption. Importantly, Ce-UiO-66-NH2@LS exhibited a removal efficiency of > 80 % after four cycles of continuous reuse. This study therefore provides a feasible method for improving the recovery capacities and adsorption performances of MOFs.
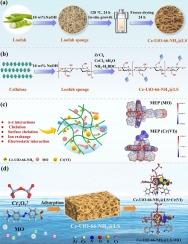
新型双金属UiO-66丝瓜海绵吸附剂的原位制备:批量实验和DFT计算
偶氮染料和重金属离子对人类生态系统的有害影响仍然是一个持续存在的问题,因此开发去除它们的方法是一个日益增长的研究领域。本研究采用原位生长法制备了具有多位点吸附功能的Ce-UiO-66-NH2@LS复合材料(LS =丝瓜海绵)。LS载体与金属有机骨架(MOF)的结合可以有效地去除甲基橙(MO)和六价铬(Cr(VI))。在低pH条件下,MO和Cr(VI)的最大吸附量分别为930.58和282.77 mg/g,去除率分别为96.70和94.90 %。在多种阴离子存在下,Ce-UiO-66-NH2@LS对MO和Cr(VI)的吸附表现出较强的抗离子干扰能力,并在MO - Cr(VI)混合体系中保持较高的吸附性能。采用四种吸附等温线和动力学模型对吸附过程进行了评价,得出化学吸附是主要吸附过程。利用x射线光电子能谱和密度泛函理论进一步分析了吸附机理。因此,发现电子相互作用和配位效应是吸附的主要机制。重要的是,Ce-UiO-66-NH2@LS在连续重复使用四个循环后的去除效率为 >; 80 %。因此,本研究为提高mof的回收能力和吸附性能提供了一种可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


