The Use of Ni(cod)(dq) (COD: 1,5-Cyclooctadiene; DQ: Duroquinone) for the Dehalogenative Coupling Polycondensation to π-Conjugated Polyarylenes
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Ni(cod)(dq) (COD: 1,5-cyclooctadiene; DQ: duroquinone) with an appropriate bipyridine ligand is available for the dehalogenative polycondensation of dihaloarenes to afford π-conjugated polymers. The reaction of 2,7-dibromo-9,9-di(n-hexylfluorene) with a nickel complex composed of Ni(cod)(dq) and 4,4′-di-tert-butyl-2,2′-bipyridine in DMF/toluene (1:4) proceeded at 120 °C for 72 h. Polyfluorene was obtained in 81% yield with Mn = 13,000 (Mw/Mn = 2.0). Ni(cod)(dq) was found to be stored under an ambient atmosphere at room temperature, the use of which after several months vs a freshly opened nickel complex resulted to afford the corresponding polymer in a comparable yield and molecular weight. Other dihaloarenes such as fluorene with different alkyl chain structures, 1,4- and 1,3-dihalobenzenes, 5,5′-dibromo-2,2′-bithiophene, and 2,6-dibromo-cyclopentadithiophene, also underwent dehalogenative polymerization to afford the corresponding conjugated polymers in excellent yields with an appropriate degree of polymerization.
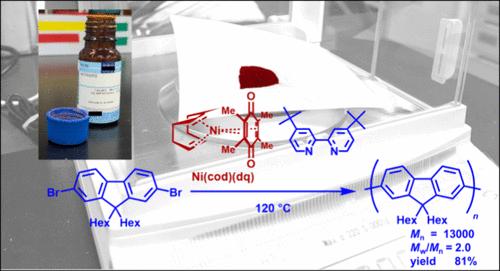
Ni(cod)(dq)(COD:1,5-环辛二烯;DQ:杜洛醌)与适当的联吡啶配体可用于二卤代烯烃的脱卤缩聚,从而得到π-共轭聚合物。2,7-dibromo-9,9-di(n-hexylfluorene) 与由 Ni(cod)(dq)和 4,4′-di-ter-t-butyl-2,2′-bipyridine 组成的镍配合物在 DMF/甲苯(1:4)中的反应在 120 °C 下进行了 72 小时。发现 Ni(cod)(dq)可在室温的环境气氛下储存,几个月后使用它与新打开的镍络合物进行对比,可得到相应的聚合物,产率和分子量相当。其他二卤烯烃,如具有不同烷基链结构的芴,1,4-和 1,3- 二卤苯,5,5′-二溴-2,2′-噻吩,以及 2,6-二溴-环戊二烯噻吩,也经过脱卤聚合反应,以适当的聚合度得到相应的共轭聚合物,产率极高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


