Building Efficient Diastereo- and Enantioselective Synthetic Routes to trans-Cyclopropyl Esters for Rapid Lead Scale-Up
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
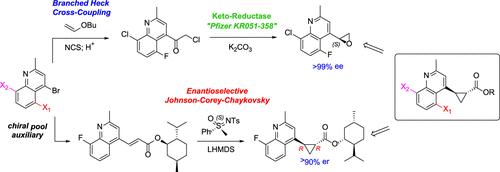
Cyclopropanes play an important role in drug discovery, and synthetic access to variedly substituted systems is an ongoing challenge for chemistry teams. A variety of scalable synthetic routes were developed and optimized for the construction of 1,2-trans-disubstituted cyclopropyl esters. The use of a stable cyclopropyl trifluoroborate provided a path for the rapid exploration of heteroaryl substituent diversity. Two asymmetric approaches were subsequently enabled as viable alternatives. Our first approach led to the development of a novel sulfoximine-driven Johnson–Corey–Chaykovsky reaction of menthyl acrylates and is the first example of this chemistry for the enantio- and diastereostereoselective construction of trans-cyclopropanes. Ultimately, a scalable process route was fashioned through the optimization of an efficient ring opening/intramolecular C–O phosphate transfer and displacement cascade that builds the trans-cyclopropyl ester from a chiral epoxide with excellent stereocontrol.
建立高效的非映对和对映选择性反式环丙酯合成路线,用于快速铅放大
环丙烷在药物发现中发挥着重要作用,对化学团队来说,合成各种取代体系是一个持续的挑战。开发和优化了多种可扩展的合成路线,以构建1,2-反式二取代环丙基酯。稳定的三氟硼酸环丙基的使用为快速探索杂芳基取代基的多样性提供了途径。随后,两种非对称方法成为可行的替代方案。我们的第一种方法导致了一种新的亚砜驱动的Johnson-Corey-Chaykovsky反应的发展,并且是这种化学对映和非对映立体选择性构造反式环丙烷的第一个例子。最终,通过优化有效的开环/分子内C-O磷酸转移和置换级联,形成了一条可扩展的工艺路线,该级联从具有良好立体控制的手性环氧化物中生成反式环丙基酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


