Microneedles Loaded with Nitric-Oxide Driven Nanomotors Improve Force-Induced Efferocytosis Impairment and Sterile Inflammation by Revitalizing Macrophage Energy Metabolism
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mechanical force initiates sterile inflammation, a process implicated in diverse physiological and pathological processes. The timely clearance of apoptotic cells by macrophages via efferocytosis is crucial for the proper resolution of sterile inflammation and for averting excessive tissue damage. Despite this, the specific role and underlying mechanisms of mechanical force on macrophage efferocytosis remain obscure. By integrating bioinformatics and metabolomics analyses, we uncovered how mechanical force disrupts the “arginine metabolism─TCA cycle─mitochondrial function” metabolic cascade, thereby impairing macrophage efferocytosis and intensifying sterile inflammation. Notably, we discovered that elevating l-arginine levels can ameliorate these crises by restoring energy metabolism. Leveraging this insight, we engineered a microneedle drug delivery system loaded with nitric-oxide driven nanomotors (MSN-LA@MNs) for targeted delivery of l-arginine. The active component, MSN-LA, exploits the heightened expression of inducible nitric oxide synthase (iNOS) in force-loaded tissues as a chemoattractant, harnessing NO generated from iNOS-catalyzed l-arginine for autonomous propulsion. In a force-induced rat orthodontic tooth movement (OTM) model, we confirmed that MSN-LA@MNs enhance macrophage efferocytosis and, under iNOS guidance, dynamically modulate sterile inflammation levels in OTM, thus facilitating the OTM process. Collectively, our findings elucidate previously unclear mechanistic links between force, macrophage efferocytosis, and sterile inflammation from a metabolic vantage point, offering a promising targeted strategy for modulating force-related biological processes such as OTM.
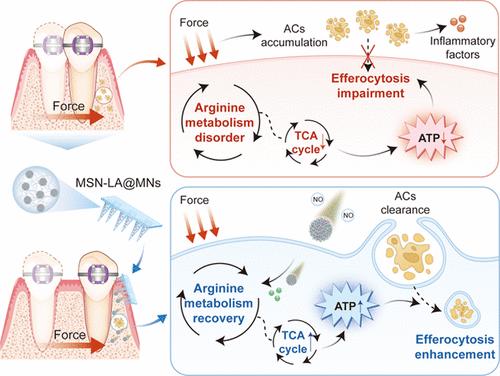
装载一氧化氮驱动纳米马达的微针通过振兴巨噬细胞的能量代谢改善力诱导的吞噬功能障碍和无菌性炎症
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


