Chimeric origins and dynamic evolution of central carbon metabolism in eukaryotes
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
The origin of eukaryotes was a key event in the history of life. Current leading hypotheses propose that a symbiosis between an asgardarchaeal host cell and an alphaproteobacterial endosymbiont represented a crucial step in eukaryotic origin and that metabolic cross-feeding between the partners provided the basis for their subsequent evolutionary integration. A major unanswered question is whether the metabolism of modern eukaryotes bears any vestige of this ancestral syntrophy. Here we systematically analyse the evolutionary origins of the eukaryotic gene repertoires mediating central carbon metabolism. Our phylogenetic and sequence analyses reveal that this gene repertoire is chimeric, with ancestral contributions from Asgardarchaeota and Alphaproteobacteria operating predominantly in glycolysis and the tricarboxylic acid cycle, respectively. Our analyses also reveal the extent to which this ancestral metabolic interplay has been remodelled via gene loss, transfer and subcellular retargeting in the >2 billion years since the origin of eukaryotic cells, and we identify genetic contributions from other prokaryotic sources in addition to the asgardarchaeal host and alphaproteobacterial endosymbiont. Our work demonstrates that, in contrast to previous assumptions, modern eukaryotic metabolism preserves information about the nature of the original asgardarchaeal–alphaproteobacterial interactions and supports syntrophy scenarios for the origin of the eukaryotic cell. Analysis of the eukaryotic gene repertoires mediating central carbon metabolism identifies ancestral contributions from Alphaproteobacteria, Asgardarchaeota and other microbial taxa, followed by gene loss, transfer and subcellular retargeting, which have remodelled central carbon metabolism over time.

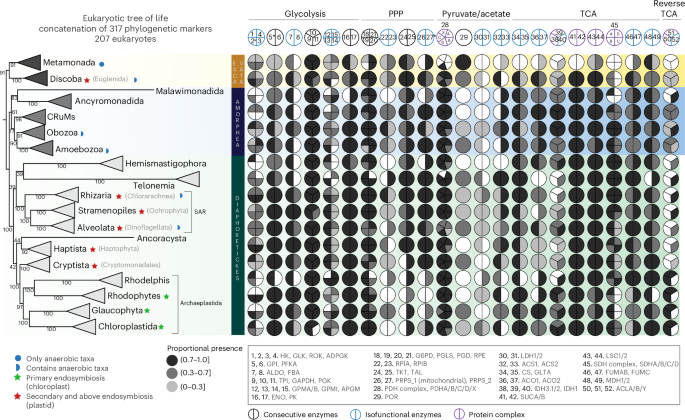
真核生物中心碳代谢的嵌合起源和动态进化
真核生物的起源是生命史上的一个关键事件。目前的主流假设认为,蛔虫宿主细胞与α变形菌内共生体之间的共生关系是真核起源的关键一步,伙伴之间的代谢交叉喂养为它们随后的进化整合提供了基础。一个悬而未决的主要问题是,现代真核生物的新陈代谢是否有这种祖先合胞的痕迹。在这里,我们系统地分析了真核生物基因库介导中央碳代谢的进化起源。我们的系统发育和序列分析表明,该基因库是嵌合的,其祖先贡献分别来自Asgardarchaeota和Alphaproteobacteria,它们主要参与糖酵解和三羧酸循环。我们的分析还揭示了自真核细胞起源以来的20亿年里,这种祖先的代谢相互作用在多大程度上通过基因丢失、转移和亚细胞重靶向进行了重塑,并且我们确定了除asgardarchaal宿主和α变形菌内共生体外,其他原核生物来源的遗传贡献。我们的工作表明,与之前的假设相反,现代真核代谢保留了有关原始asgardarchaeal - α变形菌相互作用性质的信息,并支持真核细胞起源的合胞假说。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


