A designed synthetic microbiota provides insight to community function in Clostridioides difficile resistance
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
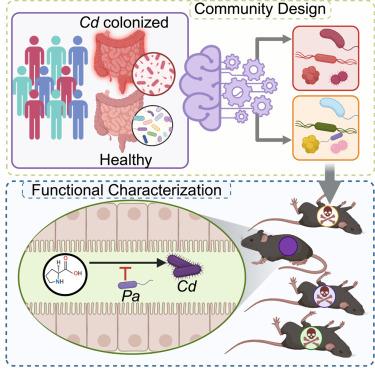
Clostridioides difficile, a major cause of antibiotic-associated diarrhea, is suppressed by the gut microbiome, but the precise mechanisms are not fully described. Through a meta-analysis of 12 human studies, we designed a synthetic fecal microbiota transplant (sFMT1) by reconstructing microbial networks negatively associated with C. difficile colonization. This lab-built 37-strain consortium formed a functional community suppressing C. difficile in vitro and in animal models. Using sFMT1 as a tractable model system, we find that bile acid 7α-dehydroxylation is not a determinant of sFMT1 efficacy while one strain performing Stickland fermentation—a pathway of competitive nutrient utilization—is both necessary and sufficient for the suppression of C. difficile, replicating the efficacy of a human fecal transplant in a gnotobiotic mouse model. Our data illustrate the significance of nutrient competition in suppression of C. difficile and a generalizable approach to interrogating complex community function through robust methods to leverage publicly available sequencing data.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


