Realigned transsulfuration drives BRAF-V600E-targeted therapy resistance in melanoma
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
BRAF V600E-inhibition effectively treats melanoma, but acquired resistance rapidly develops. Protein expression profiles, mitochondrial energetics, metabolomics and fluxomics data in cell line, xenograft, and patient-derived xenograft systems revealed that concerted reprogramming of metabolic pathways (including glutaminolysis, glycolysis, TCA cycle, electron transport chain [ETC], and transsulfuration), along with an immediate cytoprotective response to drug-induced oxidative stress, underpins drug-tolerant persister cancer cell survival. Realignment of cysteine (Cys) metabolism, in particular an immediate upregulation of cystathionine-γ-lyase (CSE), was vital in persister cells. The oxidative cellular environment, drug-induced elevated cystine uptake and oxidative Cys catabolism, increased intracellular cystine/Cys ratios, thereby favoring cystine as a CSE substrate. This produces persulfides and hydrogen sulfide to protect protein thiols and support elevated energy demand in persister cells. Combining BRAF V600E inhibitors with CSE inhibitors effectively diminished proliferative relapse in culture models and increased progression-free survival of xenografted mice. This, together with induced CSE expression in patient samples under BRAF-V600E-inhibition, reveals an approach to increase BRAF-V600E-targeted therapeutic efficacy.
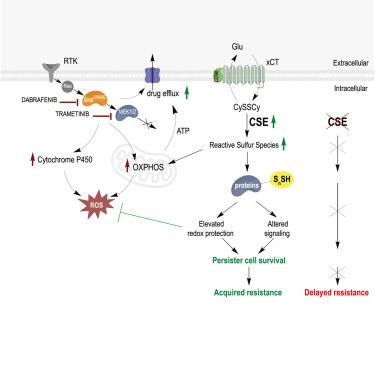
重组转硫驱动braf - v600e靶向治疗在黑色素瘤中的耐药性
BRAF v600e抑制可有效治疗黑色素瘤,但获得性耐药迅速发展。细胞系、异种移植物和患者来源的异种移植物系统中的蛋白质表达谱、线粒体能量学、代谢组学和通量组学数据显示,代谢途径(包括谷氨酰胺解解、糖酵解、TCA循环、电子传递链[ETC]和转硫)的协同重编程,以及对药物诱导的氧化应激的即时细胞保护反应,是耐药持续性癌细胞存活的基础。在持久性细胞中,半胱氨酸(Cys)代谢的重新调整,特别是半胱硫氨酸-γ-裂解酶(CSE)的立即上调,是至关重要的。氧化细胞环境,药物诱导的胱氨酸摄取和氧化胱氨酸分解代谢升高,细胞内胱氨酸/胱氨酸比值增加,因此有利于胱氨酸作为CSE底物。这会产生过硫化物和硫化氢,以保护蛋白质硫醇,并支持持久性细胞中能量需求的增加。BRAF V600E抑制剂与CSE抑制剂联合使用可有效减少培养模型中的增殖性复发,提高异种移植小鼠的无进展生存期。这与braf - v600e抑制下患者样本中诱导CSE表达一起,揭示了一种提高braf - v600e靶向治疗效果的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


