Bacterial immunotherapy leveraging IL-10R hysteresis for both phagocytosis evasion and tumor immunity revitalization
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Bacterial immunotherapy holds promising cancer-fighting potential. However, unlocking its power requires a mechanistic understanding of how bacteria both evade antimicrobial immune defenses and stimulate anti-tumor immune responses within the tumor microenvironment (TME). Here, by harnessing an engineered Salmonella enterica strain with this dual proficiency, we unveil an underlying singular mechanism. Specifically, the hysteretic nonlinearity of interleukin-10 receptor (IL-10R) expression drives tumor-infiltrated immune cells into a tumor-specific IL-10Rhi state. Bacteria leverage this to enhance tumor-associated macrophages producing IL-10, evade phagocytosis by tumor-associated neutrophils, and coincidently expand and stimulate the preexisting exhausted tumor-resident CD8+ T cells. This effective combination eliminates tumors, prevents recurrence, and inhibits metastasis across multiple tumor types. Analysis of human samples suggests that the IL-10Rhi state might be a ubiquitous trait across human tumor types. Our study unveils the unsolved mechanism behind bacterial immunotherapy’s dual challenge in solid tumors and provides a framework for intratumoral immunomodulation.
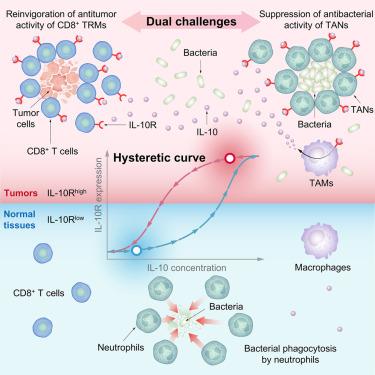
利用IL-10R滞后性进行吞噬逃避和肿瘤免疫恢复的细菌免疫疗法
细菌免疫疗法具有抗癌潜力。然而,解锁其功能需要对细菌如何逃避抗微生物免疫防御和刺激肿瘤微环境(TME)内的抗肿瘤免疫反应有一个机制的理解。在这里,通过利用这种双重熟练的工程肠沙门氏菌菌株,我们揭示了一个潜在的单一机制。具体来说,白细胞介素-10受体(IL-10R)表达的滞后非线性驱动肿瘤浸润的免疫细胞进入肿瘤特异性IL-10Rhi状态。细菌利用这一点增强产生IL-10的肿瘤相关巨噬细胞,逃避肿瘤相关中性粒细胞的吞噬,同时扩大和刺激先前存在的耗尽的肿瘤驻留CD8+ T细胞。这种有效的组合可以消除肿瘤,防止复发,并抑制多种肿瘤类型的转移。对人类样本的分析表明,IL-10Rhi状态可能是人类肿瘤类型的普遍特征。我们的研究揭示了细菌免疫治疗在实体瘤中的双重挑战背后未解决的机制,并为瘤内免疫调节提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


