A global perspective on research advances and future challenges in Friedreich ataxia
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
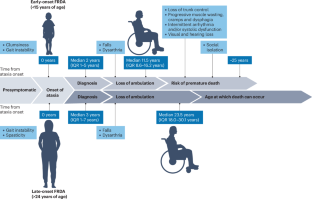
Friedreich ataxia (FRDA) is a rare multisystem, life-limiting disease and is the most common early-onset inherited ataxia in populations of European, Arab and Indian descent. In recent years, substantial progress has been made in dissecting the pathogenesis and natural history of FRDA, and several clinical trials have been initiated. A particularly notable recent achievement was the approval of the nuclear factor erythroid 2-related factor 2 activator omaveloxolone as the first disease-specific therapy for FRDA. In light of these developments, we review milestones in FRDA translational and clinical research over the past 10 years, as well as the various therapeutic strategies currently in the pipeline. We also consider the lessons that have been learned from failed trials and other setbacks. We conclude by presenting a global roadmap for future research, as outlined by the recently established Friedreich’s Ataxia Global Clinical Consortium, which covers North and South America, Europe, India, Australia and New Zealand. Friedreich ataxia (FRDA) is a rare multisystem disorder and is the most common early-onset hereditary ataxia in populations of European, Arab and Indian descent. This article reviews the milestones in FRDA translational and clinical research over the past 10 years and outlines the priorities for future FRDA research.

弗里德赖希共济失调研究进展及未来挑战的全球视角
弗里德里希共济失调(FRDA)是一种罕见的多系统、限制生命的疾病,是欧洲、阿拉伯和印度血统人群中最常见的早发性遗传性共济失调。近年来,在剖析FRDA发病机制和自然史方面取得了实质性进展,并开展了多项临床试验。最近一个特别值得注意的成就是核因子红细胞2相关因子2激活剂omaveloxolone被批准为FRDA的首个疾病特异性治疗药物。鉴于这些发展,我们回顾了过去10年来FRDA转化和临床研究的里程碑,以及目前正在开发的各种治疗策略。我们还考虑从失败的试验和其他挫折中吸取的教训。最后,我们提出了未来研究的全球路线图,正如最近成立的弗里德赖希共济失调全球临床联盟所概述的那样,该联盟涵盖北美和南美、欧洲、印度、澳大利亚和新西兰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


