Hepatic stellate cells regulate liver fatty acid utilization via plasmalemma vesicle-associated protein
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The liver is essential for normal fatty acid utilization during fasting. Circulating fatty acids are taken up by hepatocytes and esterified as triacylglycerols for either oxidative metabolization and ketogenesis or export. Whereas the regulation of fatty acid oxidation in hepatocytes is well understood, the uptake and retention of non-esterified fatty acids by hepatocytes is not. Here, we show that murine hepatic stellate cells (HSCs) and their abundantly expressed plasmalemma vesicle-associated protein (PLVAP) control hepatic substrate preference for fasting energy metabolism. HSC-specific ablation of PLVAP in mice elevated hepatic insulin signaling and improved glucose tolerance. Fasted HSC PLVAP knockout mice showed suppressed hepatic fatty acid esterification into di- and triacylglycerols, shifting fasting metabolism from fatty acid oxidation to reliance on carbohydrates. By super-resolution microscopy, we localized HSC PLVAP to caveolae residing along the sinusoidal lumen, supporting a role for HSCs and PLVAP-diaphragmed caveolae in normal fasting metabolism of the liver.
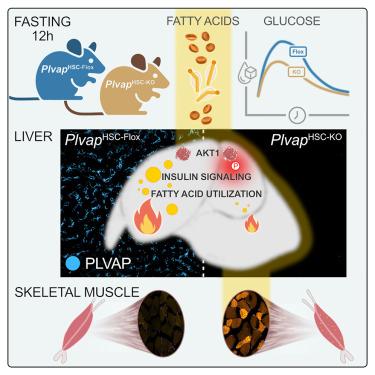
肝星状细胞通过质膜囊泡相关蛋白调节肝脏对脂肪酸的利用
在禁食期间,肝脏对正常的脂肪酸利用是必不可少的。循环脂肪酸被肝细胞吸收并酯化为三酰基甘油,用于氧化代谢和生酮或输出。虽然脂肪酸氧化在肝细胞中的调控已被很好地理解,但肝细胞对非酯化脂肪酸的摄取和保留尚不清楚。本研究表明,小鼠肝星状细胞(hsc)及其丰富表达的质膜囊泡相关蛋白(PLVAP)控制着肝脏底物对空腹能量代谢的偏好。hsc特异性消融小鼠PLVAP可升高肝脏胰岛素信号并改善葡萄糖耐量。禁食的HSC PLVAP敲除小鼠显示肝脏脂肪酸酯化成二甘油酯和三甘油酯受到抑制,将空腹代谢从脂肪酸氧化转变为对碳水化合物的依赖。通过超分辨率显微镜,我们将HSC PLVAP定位到位于窦腔的小窝中,支持HSC和PLVAP膜小窝在肝脏正常空腹代谢中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


