Carbamazepine-exposed earthworms are characterized by tissue-specific accumulation patterns and transcriptional profiles
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Pharmaceutically active compounds enter soils via wastewater reuse and biosolid application. A ubiquitous drug present in wastewater is carbamazepine, a frequently prescribed anti-convulsant. Its mode of action is not species-specific and affects the nervous system of non-target organisms, including most likely the soil dwelling earthworms, which in turn has the potential to negatively impact soil quality. In this project, soils were amended with carbamazepine to explore uptake dynamics and resultant changes in molecular and life cycle endpoints of earthworms (Dendrobaena veneta). Earthworms were maintained, under laboratory conditions, for 28 days in soil spiked with either a solvent control, 0.6 mg/kg carbamazepine (encountered in the terrestrial system) or 10 mg/kg carbamazepine (significantly above an environmental hotspot). Carbamazepine concentrations were quantified in soils and worms by liquid chromatography tandem mass spectrometry (LC-MS/MS) which revealed tissue, dose and time-dependent differences in accumulation. Carbamazepine also modulated the make-up of the microbiome in the soil as well as the earthworm’s gut. De novo RNA sequencing identified novel transcripts and complex tissue-specific transcriptomic changes, where, for example, the expression of the tubulin polymerisation promoting protein (tppp) was inhibited (9-fold) in the gut but induced (11-fold) in the cerebral ganglion of exposed earthworms. However, the notable absence of a strong cytochrome P450 response across all conditions suggests that the terrestrial earthworm also relies on detoxification pathways that differ to those observed in well-studied aquatic models. The novel finding that carbamazepine exposure triggers tissue-specific impacts in non-target soil organisms highlights the value and need for a more comprehensive understanding of how contaminants of emerging concern behave within an ecotoxicological context. This, in turn, will lead to informed and reliable risk assessments defining the consequences of wastewater and biosolid amendment practices on soil ecology and ecosystem function.
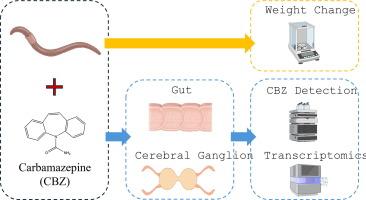
药物活性化合物通过废水回用和生物固体应用进入土壤。废水中普遍存在的一种药物是卡马西平,它是一种常用的抗惊厥药。它的作用模式不针对特定物种,会影响非目标生物的神经系统,其中很可能包括生活在土壤中的蚯蚓,进而可能对土壤质量产生负面影响。在本项目中,用卡马西平对土壤进行改良,以探索蚯蚓(Dendrobaena veneta)对卡马西平的吸收动态及其分子和生命周期终点的变化。在实验室条件下,蚯蚓在添加了溶剂对照、0.6 毫克/千克卡马西平(在陆地系统中出现)或 10 毫克/千克卡马西平(明显高于环境热点)的土壤中维持 28 天。采用液相色谱串联质谱法(LC-MS/MS)对土壤和蠕虫中的卡马西平浓度进行了定量分析,结果表明其积累随组织、剂量和时间的变化而变化。卡马西平还调节了土壤和蚯蚓肠道中微生物群的组成。从头开始的 RNA 测序发现了新的转录本和复杂的组织特异性转录组变化,例如,在暴露的蚯蚓肠道中,小管聚合促进蛋白 (tppp) 的表达受到抑制(9 倍),但在脑神经节中却受到诱导(11 倍)。然而,在所有条件下都没有出现强烈的细胞色素 P450 反应,这表明陆生蚯蚓也依赖于解毒途径,这种途径不同于在研究充分的水生模型中观察到的途径。卡马西平暴露会对非目标土壤生物的组织产生特定影响,这一新颖发现突出表明了更全面地了解新关注污染物在生态毒理学背景下的行为方式的价值和必要性。反过来,这将有助于进行知情、可靠的风险评估,确定废水和生物固体修正方法对土壤生态和生态系统功能的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


