Synthesis of N-Heterocycle-Ligated Porphyrins Using Iodobenzene Diacetate Enabled Regioselective Cross-Dehydrogenation of Porphyrins and NH-Heteroaromatics
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
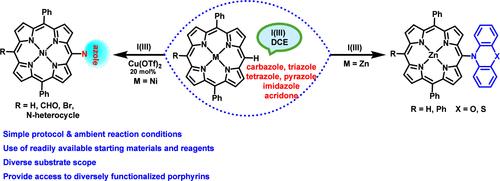
Preparation of diverse meso-functionalized porphyrins involves iodine(III)- and copper triflate-promoted dehydrogenative coupling of meso-free porphyrins and appropriate NH-free heterocycles. Reaction conditions involving the stable and recyclable iodobenzene diacetate reagent are compatible with a range of NH-free heterocycles (acridone, phenoxazine and phenothiazine, carbazole, β-carbolin triazoles, imidazole, pyrazole, indazole, and tetrazole) and porphyrins to access diversely functionalized A3B, A2BC, and A2B2 porphyrins in moderate to good yields. The prepared heterocycle-appended porphyrins exhibit modestly red-shifted Soret and Q bands in the absorption spectra.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


