Enzyme Reactions Are Accelerated or Decelerated When the Enzymes Are Located Near the DNA Nanostructure
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
It is known experimentally that enzymatic reactions are often accelerated when the enzymes are assembled on the scaffold of DNA nanostructures. However, the exact mechanism by which this acceleration occurs remains unclear. Here, we study the reactions of enzymes with different catalytic mechanisms assembled on a DNA scaffold with various substrates. Analysis of the hydration properties of the substrates using our accurate statistical mechanics theory classifies the substrates into two groups that behave as hydrophilic and hydrophobic solutes, respectively. The reaction of the enzyme on the DNA scaffold is accelerated with a hydrophilic substrate but decelerated with a hydrophobic substrate. We propose a mechanism of acceleration or deceleration in which, due to the formation of a high-density layer of water near the DNA surface with high negative charge density, the concentration of a substrate with high energetic affinity for water within the layer becomes higher than that near a free enzyme, whereas that of a substrate with low energetic affinity becomes lower within the layer. This study provides chemical and physical insights into a general case of biocatalysts, where the rates of chemical reactions occurring at the interface of biomolecules in aqueous environments can differ substantially from those in the bulk solution due to variations in the local concentration of a given ligand.
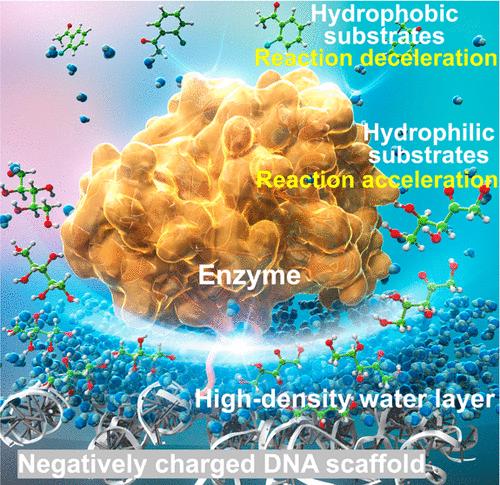
实验表明,当酶组装在 DNA 纳米结构的支架上时,酶反应通常会加速。然而,这种加速反应发生的确切机制仍不清楚。在这里,我们研究了装配在 DNA 支架上的具有不同催化机制的酶与各种底物的反应。我们利用精确的统计力学理论分析了底物的水合特性,将底物分为两类,分别表现为亲水性溶质和疏水性溶质。亲水性底物会加速酶在 DNA 支架上的反应,而疏水性底物则会减慢反应速度。我们提出了一种加速或减速机制,即由于在 DNA 表面附近形成了高负电荷密度的高密度水层,水层内对水具有高能亲和力的底物的浓度会高于游离酶附近的浓度,而水层内对水具有低能亲和力的底物的浓度则会降低。这项研究为生物催化剂的一般情况提供了化学和物理方面的见解。在水环境中,由于给定配体的局部浓度变化,生物分子界面上发生的化学反应速率可能与主体溶液中的化学反应速率大不相同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


