Selective removal and utilization of copper from electroplating wastewater by modified MXene-based capacitive deionization
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Capacitive deionization (CDI) technology has promising potential for the removal of copper ions from electroplating wastewater. In this study, a new kind of FeS2 modified MXene electrode material with three-dimensional mutual support structure was synthesized, which has excellent hydrophilicity, electrical conductivity, and specific redox activity for copper ions. Therefore, this material was applied for the selective removal of copper ions from electroplating wastewater through the use of CDI. The FeS2/MXene electrode demonstrated excellent electrosorption performance, with an electrosorption capacity (EC) of 80.02 mg g−1 and a removal efficiency of 96.02 %. The electrosorption mechanism involves three synergistic pathways: (1) Faradaic redox reactions between Fe2+/Fe3+ and Cu2+/Cu+, (2) ion intercalation, and (3) capacitive adsorption. Notably, the material exhibited remarkable selectivity for Cu2+ even in mixed ion systems, with distribution coefficients exceeding 104 mL g−1. Additionally, Cu2+ in the actual electroplating wastewater was efficiently removed (99.97 %), meeting the water quality standard for metal plating and chemical coating process. Furthermore, the electrosorption-saturated electrodes with copper ions were reused as raw materials to prepare Cu@FeS2/MXene electrocatalysts for nitrate (NO3–-N) reduction. The electrocatalytic efficiency of NO3–-N was 86.9 %, and the selectivity for nitrogen (N2) reached 98.3 %. This study opens up a promising approach not only for selectively removing copper from wastewater but also for utilizing copper-saturated electrodes, demonstrating a sustainable and efficient strategy for water treatment and resource recovery.
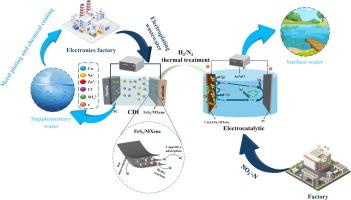
利用改良的基于 MXene 的电容式去离子法选择性去除和利用电镀废水中的铜
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


