Spatially separated MnOx/Pt dual co-catalysts on CdS hollow spheres with ultrafast carrier transfer kinetics
IF 14.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
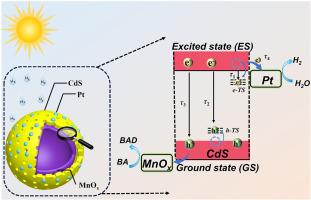
Dual co-catalyst loading is a viable strategy to enhance charge carrier separation in photocatalysis. However, conventional randomly-loaded dual co-catalysts often fail to effectively direct charge transfer. In this study, a strategically designed spatially separated dual co-catalyst system (MnOx/CdS/Pt) optimizes redox site orientation to address the challenge of disordered carrier transfer. This configuration maximizes the utilization of both electrons and holes while establishing ultrafast electron transfer channels between CdS and Pt. The ultrafast electron transfer channels between spatially separated redox sites are demonstrated by femtosecond transient absorption (fs-TA) spectroscopy and in situ characterization. The average lifetime of MnOx/CdS/Pt (MCSP) in a real reaction environment reduced from ∼ 1352.6 to ∼ 996.6 ps, compared to CdS alone. The interfacial electron transfer rate is accelerated to ∼ 2.6 × 108 s−1, a substantial improvement over the CdS/Pt (∼ 6.0 × 107 s−1). Consequently, this system achieves efficient hydrogen production coupled with fine chemical synthesis. This work underscores the potential of rational dual co-catalyst design with spatially separated redox sites as a promising strategy for developing high-performance photocatalytic platforms for solar fuel production.
具有超快载流子转移动力学的 CdS 空心球上空间分离的氧化锰/铂双助催化剂
双助催化剂负载是光催化中增强载流子分离的可行策略。然而,传统的随机负载双助催化剂往往不能有效地引导电荷转移。在这项研究中,战略性地设计了空间分离的双助催化剂体系(MnOx/CdS/Pt),优化了氧化还原位点的取向,以解决无序载体转移的挑战。这种结构最大限度地利用了电子和空穴,同时在CdS和Pt之间建立了超快电子转移通道。飞秒瞬态吸收(fs-TA)光谱和原位表征证明了空间分离的氧化还原位点之间的超快电子转移通道。与单独使用CdS相比,MnOx/CdS/Pt (MCSP)在真实反应环境中的平均寿命从~ 1352.6 ps降低到~ 996.6 ps。界面电子转移速率加快到~ 2.6 × 108 s−1,比CdS/Pt(~ 6.0 × 107 s−1)有了实质性的提高。因此,该系统实现了高效的制氢和精细的化学合成。这项工作强调了具有空间分离氧化还原位点的合理双共催化剂设计的潜力,作为开发用于太阳能燃料生产的高性能光催化平台的有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


