Sol-gel derived bismuth-doped nickel ferrite: A promising adsorbent to tackle Cr(VI) pollution – Insights into the thermodynamics, kinetics, and isotherms
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
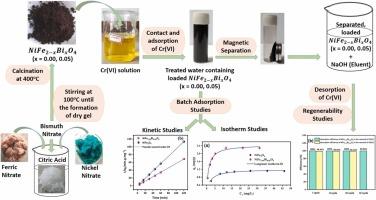
Bismuth-doped nickel ferrite nanoparticles (NiFe2-xBixO4 with x = 0.00, 0.03, 0.05) were synthesized using the sol-gel autocombustion method. The optimum bismuth doping concentration for enhanced Cr(VI) adsorption is determined to be x = 0.05. XRD analysis confirmed a mixed spinel structure for the synthesized ferrites and revealed that bismuth doping results in decreased crystallite size, increased surface area, and enhanced dislocation density. Batch adsorption studies showed improved removal efficiency with an increase in adsorbent dosage and contact time, but decreased efficiency with an increase in pH, temperature, and initial ion concentration. The introduction of Bi3+ ions increased the pHpzc of NiFe2O4 from 6.21 to 7.18. Thermodynamic analysis showed that the adsorption process is spontaneous at lower temperatures but non-spontaneous at and above 318 K for NiFe2O4 and 333 K for NiFe1.95Bi0.05O4. Adsorption kinetics studies reveal that the adsorption rate follows pseudo-second-order kinetics, indicating a second-order process with chemical adsorption mechanisms. Diffusion kinetic model analysis shows that Cr(VI) adsorption occurs via boundary layer diffusion. Isotherm data fit well with the Langmuir model, suggesting monolayer adsorption. Under optimized conditions (pH 1, temperature 273K, contact time 45 min), 2 g/L NiFe2-xBixO4 (x = 0.00, 0.05) at 300 rpm achieved 80.698 % (NiFe2O4) and 92.188 % (NiFe1.95Bi0.05O4) Cr(VI) removal, with adsorption capacities 40.349 and 46.094 mg/g, respectively. Regenerability studies showed desorption efficiency exceeding 98 %, allowing reuse of the adsorbents for at least 3 cycles with a negligible decrease in adsorption efficiency (<0.1 %). The study demonstrates that incorporating bismuth enhances NiFe2O4's adsorption performance, offering a promising solution for Cr(VI) removal from aqueous solutions.
溶胶凝胶衍生掺铋镍铁氧体:解决六价铬污染的前景广阔的吸附剂--对热力学、动力学和等温线的见解
采用溶胶-凝胶自燃烧法制备了掺杂铋的铁酸镍纳米颗粒(NiFe2-xBixO4, x=0.00, 0.03, 0.05)。确定了增强Cr(VI)吸附的最佳铋掺杂浓度为x=0.05。XRD分析证实了合成的铁素体具有混合尖晶石结构,并表明铋的掺杂导致晶体尺寸减小,表面积增大,位错密度增大。间歇吸附研究表明,随着吸附剂用量和接触时间的增加,去除效率提高,但随着pH、温度和初始离子浓度的增加,效率降低。Bi3+离子的引入使NiFe2O4的pHpzc由6.21提高到7.18。热力学分析表明,NiFe2O4和NiFe1.95Bi0.05O4的吸附过程在较低温度下是自发的,而在318 K及以上温度下则是非自发的。吸附动力学研究表明,吸附速率服从准二级动力学,表明二级过程具有化学吸附机理。扩散动力学模型分析表明,Cr(VI)吸附是通过边界层扩散进行的。等温线数据与Langmuir模型吻合较好,表明是单层吸附。在优化条件(pH 1,温度273K,接触时间45 min)下,2 g/L NiFe2-xBixO4 (x=0.00, 0.05)在300 rpm下对Cr(VI)的去除率分别为80.698% (NiFe2O4)和92.188% (NiFe1.95Bi0.05O4),吸附量分别为40.349和46.094 mg/g。可再生性研究表明,解吸效率超过98%,允许吸附剂重复使用至少3个循环,而吸附效率的下降可以忽略不计(0.1%)。研究表明,添加铋可以提高NiFe2O4的吸附性能,为去除水中的Cr(VI)提供了一种有前途的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


