Sensitizing ferroptotic and apoptotic cancer therapy via tailored micelles-mediated coenzyme and ATP depletion under hypoxia
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
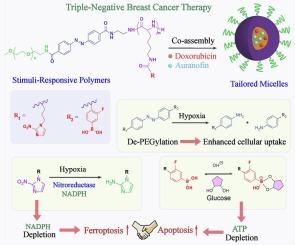
Concurrent induction of apoptosis and ferroptosis is promising in handling heterogenous cancers. We report a tailored polymeric micellar nanoplatform for the combinational anti-tumor therapy. Two stimuli-responsive amphiphlic block copolymers were synthesized, bearing three functional moieties, i.e. azobenzene, nitroimidazole and 3-fluorophenylboronic acid. Azobenzene could enhance the cellular uptake of micelles. Nitroimidazole and 3-fluorophenylboronic acid could deplete the reduced nicotinamide adenine dinucleotide phosphate (NADPH), glucose and adenosine triphosphate (ATP) under hypoxia, sensitizing ferroptotic and apoptotic cell death. The proof-of-concept was demonstrated in a triple-negative breast cancer cell line (MDA-MB-231). Irrespective of the free or encapsulated form, doxorubicin and auranofin showed a synergistic action, evidenced by a low combination index (< 1). The co-delivery micelles showed improved potency than the single drug-loaded micelles in terms of the biomarkers of apoptosis (e.g. caspase 3/9, cytochrome c and ATP) and ferroptosis (e.g. thioredoxin reductase, thioredoxin, glutathione, NADPH, malondialdehyde and lipid peroxides). The apoptosis and ferroptosis induction ability of cargo-free micelles was proved. The in vivo efficacy was verified in the MDA-MB 231 tumor-bearing nude mice model. The current work offers a promising strategy of combinational anti-tumor drug delivery for potent induction of ferroptosis and apoptosis via the sensitization effect of vehicle in the hypoxic tumor.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


