Regioselective Pyrazole Synthesis via Base-Mediated [3+2] Cycloaddition of 2-Alkynyl-1,3-Dithianes and Sydnones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present a novel base-mediated [3+2] cycloaddition for the regioselective synthesis of polysubstituted pyrazoles using 2-alkynyl-1,3-dithianes and sydnones. By exploiting the umpolung and nucleophilic properties of 2-alkynyl-1,3-dithianes, this method achieves efficient pyrazole construction under mild conditions with excellent regioselectivity, broad functional group tolerance, and diverse substrate compatibility. Furthermore, the unique reactivity of the dithianyl group enables facile derivatization and synthesis of highly functionalized pyrazoles.
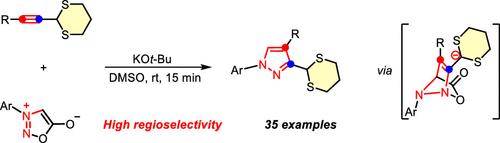
碱基介导[3+2]环加成2-烷基-1,3-二硫烷和酮类化合物合成区域选择性吡唑
我们提出了一种新的碱基介导的[3+2]环加成反应,用于使用2-炔基-1,3-二硫烷和西酮进行区域选择性合成多取代吡唑。该方法利用2-炔基-1,3-二硫烷的亲核性质,在温和的条件下实现了吡唑的高效构建,具有优异的区域选择性、广泛的官能团耐受性和多种底物相容性。此外,二硫酰基的独特反应性使得衍生化和高功能化吡唑的合成变得容易。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


