Isothiazole Construction by Metal-Free Reactions of Thioamides with Iminoiodinanes and Their Photophysical Properties
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
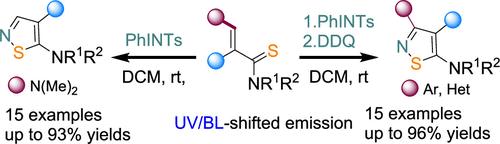
Metal-free reactions of thioacetamide derivatives with N-sulfonyliminoiodinane, affording isothiazoles under mild conditions, are described. 2-Substituted thioacetamides modified with an enamine moiety undergo reactions with iminoiodinane to form 4,5-disubstituted isothiazoles in 72–93% yields. Reactions of (het)arylidene-substituted 2-thioacetamides and iminoiodinane with subsequent one-pot oxidation of the formed sulfonylisothiazolines afford 3,4,5-trisubstituted isothiazoles in 44–96% yields. A scale-up synthesis of the developed methods was performed. The photophysical properties of the new isothiazoles were investigated, and UV- or blue-light-shifted fluorescence has been detected (QY up to 11%).
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


