Collaboration between interleukin-7 and -15 enables adaptation of tissue-resident and circulating memory CD8+ T cells to cytokine deficiency
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
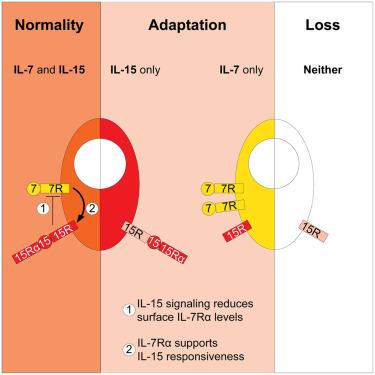
Interleukin-7 (IL-7) is considered a critical regulator of memory CD8+ T cell homeostasis. However, this is primarily based on circulating memory populations, and the cell-intrinsic requirement for IL-7 signaling during memory homeostasis has not been directly tested. Here, we addressed the role for IL-7Rα in circulating and resident memory CD8+ T cells (Trm) after their establishment. We found that inducible Il7ra deletion had only a modest effect on persistence of circulating memory and Trm subsets, causing reduced basal proliferation. Loss of IL-15 signaling imposed heightened IL-7Rα dependence on memory CD8+ T cells, including Trm cells described as IL-15 independent. In the absence of IL-15 signaling, IL-7Rα was elevated, and loss of IL-7Rα signaling reduced IL-15-elicited proliferation, suggesting crosstalk between these pathways in memory CD8+ T cells. Thus, across subsets and tissues, IL-7 and IL-15 act in concert to support memory CD8+ T cells, conferring resilience to altered availability of either cytokine.
白细胞介素-7和-15之间的协作使组织驻留和循环记忆CD8+ T细胞适应细胞因子缺乏
白细胞介素-7 (IL-7)被认为是记忆性CD8+ T细胞稳态的关键调节因子。然而,这主要是基于循环记忆种群,并且记忆稳态期间细胞对IL-7信号的内在需求尚未直接测试。在这里,我们研究了IL-7Rα在循环和常驻记忆CD8+ T细胞(Trm)建立后的作用。我们发现,诱导的Il7ra缺失对循环记忆和Trm亚群的持久性只有适度的影响,导致基底细胞增殖减少。IL-15信号的缺失导致IL-7Rα对记忆性CD8+ T细胞的依赖性增强,包括不依赖IL-15的Trm细胞。在缺乏IL-15信号的情况下,IL-7Rα升高,IL-7Rα信号的缺失减少了IL-15诱导的增殖,提示记忆性CD8+ T细胞中这些通路之间存在串串。因此,在整个亚群和组织中,IL-7和IL-15协同作用以支持记忆性CD8+ T细胞,赋予对任何一种细胞因子可用性改变的恢复能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


