Discovery of a Potent and in Vivo Anti-inflammatory Efficacious, P2Y14R Antagonist with a Novel Benzisoxazoles Scaffold by DNA-Encoded Chemical Library Technology
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
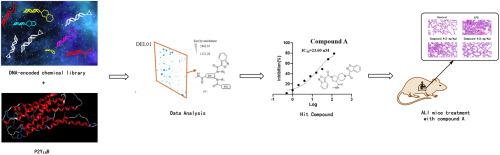
P2Y14R is activated by UDP (uridine diphosphate) and UDP glucose and associated with the development of many inflammatory diseases. P2Y14R antagonists are expected to be a new choice for the treatment of inflammatory diseases. A DNA-encoded chemical library (DEL) of 4 billion molecules was screened, leading to the identification of compound A, a novel benzisoxazole scaffold-based P2Y14 antagonist with an IC50 value of 23.60 nM. Binding mode analysis and SPR analysis (KD = 7.26 μM) demonstrated that Compound A bind strongly to P2Y14R. Molecular dynamics simulations and binding free energy calculations were performed to analyze the binding mode of Compound A with P2Y14R. And in the LPS-induced acute lung injury mice, after treatment with Compound A, the degree of lung injury was greatly reduced, the infiltration of immune cells was decreased, the level of inflammatory factors IL-6, TNF-α and IL-β were considerably decreased. Compound A exhibited good P2Y14R antagonist activity, demonstrated efficacy both in vitro and in vivo, possessed favorable druggability, and featured a novel benzisoxazole scaffold with potential for further optimization, providing a new strategy for developing subsequent P2Y14 antagonists.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


