Structure-based Design of Novel 2,4,5-Trisubstituted Pyrimidine Derivatives as Potent HIV-1 NNRTIs by Exploiting the Tolerant Regions in NNTRIs Binding Pocket
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
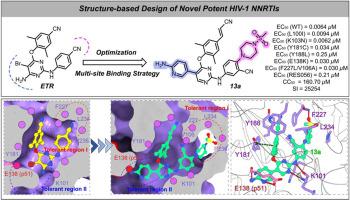
To promote the development of the new generation of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs), a series of novel 2,4,5-trisubstituted pyrimidine derivatives targeting the “tolerant region I” and “tolerant region II” of NNRTI binding pocket (NNIBP) were designed through multi-site binding strategy. Among them, 13a was demonstrated with an improved potency against wild-type (WT) and a panel of mutant HIV-1 strains with EC50 values ranging from 0.0062-0.25 μM, being superior to that of efavirenz (EFV, EC50 = 0.0080-0.37 μM). In addition, 13a was proved to have low cytotoxicity (CC50 = 160.7 μM) and high SI values (SI = 25254). Further HIV-1 RT inhibition assay demonstrated that 13a is a classical NNRTI with an IC50 value of 0.41 μM. Molecular docking and molecular dynamics simulations results illustrated its binding mode with HIV-1 RT. Overall, these enchanting results illuminated the potential of 13a as a promising lead for the development of the new generation HIV-1 NNRTIs drugs.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


