LPS-Enriched Interaction Drives Spectrum Conversion in Antimicrobial Peptides: Design and Optimization of AA16 Derivatives for Targeting Gram-Negative Bacteria
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
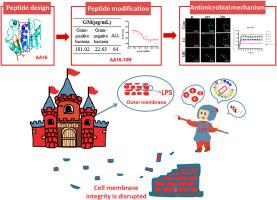
The increasing prevalence of antibiotic-resistant Gram-negative bacteria necessitates the development of novel antimicrobial agents with targeted specificity. In this study, we designed and optimized derivatives of the antimicrobial peptide AA16, which truncated from CD14 protein α-helical region, to selectively target Gram-negative bacteria by enhancing lipopolysaccharide (LPS)-enriched interactions, thereby achieving antibacterial spectrum conversion. Starting from the parent peptide AA16 (Ac-AARIPSRILFGALRVL-Amide), we performed strategic amino acid substitutions based on structure–activity relationship analysis. This led to the identification of AA16-10R, a derivative with a specific substitution at position 10, which demonstrated significantly enhanced antibacterial activity against Gram-negative strains such as Escherichia coli and Pseudomonas aeruginosa, while maintaining low hemolytic activity. Mechanistic studies revealed that AA16-10R exhibited a strong binding affinity to LPS (Kd = 0.15 μM), and its interaction with LPS induced the formation of an α-helical structure. This conformational change facilitated its accumulation on the bacterial outer membrane and disrupted membrane integrity. Our innovative approach of exploiting LPS-enriched interactions successfully converted the antimicrobial spectrum of AA16 derivatives from broad-spectrum to Gram-negative-specific. This study highlights a novel strategy for the rational design of antimicrobial peptides based on specific protein–protein interactions, offering a promising avenue for targeted antimicrobial therapy against Gram-negative pathogens.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


