Constructing a Dielectric Fluorinated Solid Electrolyte for Practically Operated All-Solid-State Lithium-Metal Batteries
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
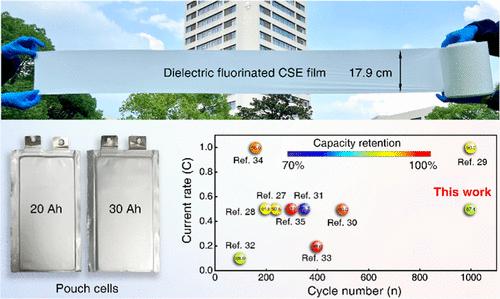
The operation of all-solid-state lithium-metal batteries is primarily constrained by an inferior solid electrolyte. Here, we employ a porous dielectric fluorinated electrolyte to encapsulate a Li+ complex, achieving rapid and stable ion conduction throughout cycling. The electrolyte comprises a porous nanofiber (NF) skeleton made of dielectric fluorinated BaTiO3 (F-BaTiO3−δ) and all-trans block copolymer PVDF-b-PTFE, with an encapsulated poly(ethylene oxide) (PEO)-LiTFSI filler. The dielectric polarized NFs effectively dissociate LiTFSI to form a rapid conductive Li+ complex, while F-BaTiO3−δ bonds with PVDF-b-PTFE and PEO to create stable cross-phase Li+-conduction paths. This results in an electrolyte with a high room-temperature conductivity of 5.64 × 10–4 S cm–1 and a low activation energy of 0.21 eV. Additionally, the polarized electrolyte achieves dynamic interface stability by eliminating the space charge layer on the cathode and internal stress on the anode. The all-solid-state LiFePO4//Li batteries can cycle stably 1000 times at 0.5 C with a high capacity retention of 87.45%. Furthermore, the NCM811//Li and 30-Ah-pouch cells also demonstrate high cycling stability, showcasing potential commercial applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


