Tetrahedral-DNA-Nanostructure-Modified Engineered Extracellular Vesicles Enhance Oral Squamous Cell Carcinomas Therapy by Targeting GPX4
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Oral squamous cell carcinoma (OSCC) represents a heterogeneous group of malignancies originating from the mucosal lining of the oral cavity. Current treatment modalities primarily involve surgery, chemotherapy, and radiotherapy. Despite the use of multimodal therapy, the 5 year overall survival rate for OSCC remains around 50%, underscoring the need for the development of nontoxic agents with potent antitumor activity. Extracellular vesicles (EVs) are nanoscale, membrane-bound structures that can selectively deliver small molecules, nucleic acids, and proteins to target cells, making them a promising platform for drug delivery in cancer therapy. Strategies to improve the uptake of EVs and enhance the delivery of therapeutic molecules to target cells are critical for advancing precision medicine. Tetrahedral DNA nanostructures (TDNs) have shown significant potential in facilitating drug endocytosis and delivery, as well as improving tissue penetration. In this study, TDN@EVs were conducted by modifying the membrane surface of M1-EVs with TDNs, which demonstrated improved biological stability and drug delivery efficiency compared to unmodified EVs. In vitro and in vivo experiments showed that TDN@EVs significantly inhibited OSCC cell proliferation and migration while promoting apoptosis. TDN@EVs exhibited superior drug penetration properties, further amplifying their antitumor effects. Proteomic analysis identified Hsc70 as the key protein responsible for the antitumor activity of the TDN@EVs. The efficient delivery of Hsc70 into tumor cells by TDN@EVs led to the degradation of GPX4, inducing ferroptosis, mitochondrial stress, and DNA damage in tumor cells. These findings highlight the potential of TDN@EVs as an effective and safe approach for cancer therapy. In conclusion, TDN@EVs present as a promising effective strategy for the targeted delivery of therapeutic agents in OSCC treatment, offering enhanced biological stability, efficient drug delivery, and significant antitumor effects.
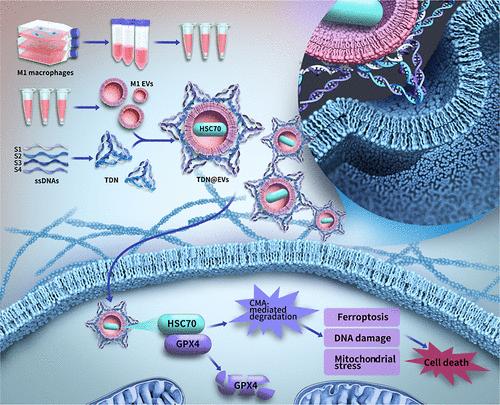
四面体dna -纳米结构修饰的工程细胞外囊泡通过靶向GPX4增强口腔鳞状细胞癌的治疗
口腔鳞状细胞癌(OSCC)是一种起源于口腔粘膜的异质性恶性肿瘤。目前的治疗方式主要包括手术、化疗和放疗。尽管使用了多模式治疗,OSCC的5年总生存率仍然在50%左右,这强调了开发具有有效抗肿瘤活性的无毒药物的必要性。细胞外囊泡(EVs)是一种纳米级的膜结合结构,可以选择性地将小分子、核酸和蛋白质递送到靶细胞,这使其成为癌症治疗中很有前途的药物递送平台。提高ev的吸收和增强治疗分子向靶细胞的递送的策略对于推进精准医疗至关重要。四面体DNA纳米结构(tdn)在促进药物内吞和递送以及改善组织渗透方面显示出巨大的潜力。在本研究中,TDN@EVs通过用tdn修饰m1 - ev的膜表面,与未修饰的ev相比,表现出更高的生物稳定性和给药效率。体外和体内实验表明,TDN@EVs显著抑制OSCC细胞增殖和迁移,促进细胞凋亡。TDN@EVs表现出优异的药物穿透性能,进一步增强了其抗肿瘤作用。蛋白质组学分析发现Hsc70是TDN@EVs抗肿瘤活性的关键蛋白。通过TDN@EVs将Hsc70高效递送到肿瘤细胞中,导致GPX4降解,诱导肿瘤细胞铁下垂、线粒体应激和DNA损伤。这些发现突出了TDN@EVs作为一种有效和安全的癌症治疗方法的潜力。综上所述,TDN@EVs是一种有前景的有效策略,可用于OSCC治疗中靶向递送治疗药物,具有增强的生物稳定性、高效的药物递送和显著的抗肿瘤作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


