Strong Metal-Support Interaction to Invert Hydrogen Evolution Overpotential of Cu Coating for High-Coulombic-Efficiency Stable Zn Anode in Aqueous Zn-Ion Batteries
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
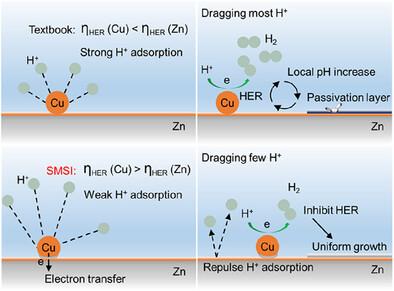
Cu exhibits strong zincophilic properties but suffers from a much lower hydrogen evolution reaction (HER) overpotential compared to Zn, which significantly undermines the coulombic efficiency and stability of the Zn anode. Consequently, Cu is regarded as an unsuitable coating for Zn anode protection. In this work, the HER overpotential of Cu versus Zn is inverted through strong metal-support interaction (SMSI) to modify the electronic structure of Cu. This interaction facilitates electron transfer, enriching positive charge and slowing down the adsorption kinetics of H+ on the Cu surface. As a result, at very low current densities of 0.2 and 2 mA cm⁻2, the Cu-coated-Zn||Cu cell achieves exceptionally high coulombic efficiencies of 99.11% and 99.91% over 2500 and 1600 h of cycling (100% depth of discharge (DOD)), which remarkably surpasses the performance of Zn anode protective coatings all reported. Moreover, a 1 Ah soft-packed full battery is not bulged and retains 94.7% of its initial capacity after 150 cycles. This study overturns the conventional concept by leveraging SMSI to tune the electronic structure, reverses the HER overpotential, and expands the range of viable metals for anode protection in aqueous metal batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


