Electrified synthesis of n-propanol using a dilute alloy catalyst
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
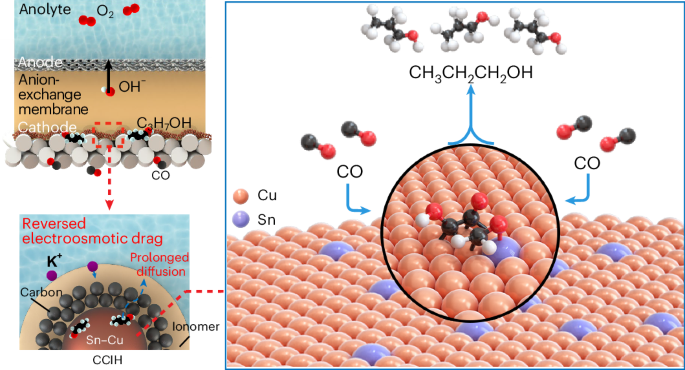
N-propanol is an important industrial solvent but the current industrial routes for its production rely on fossil fuels and generate high carbon dioxide emissions. Replacing fossil processes with electrochemical systems powered using renewable energy offers one route to reduce the carbon intensity of n-propanol manufacture. The electrosynthesis of n-propanol via carbon monoxide electroreduction relies on the coupling of C1 and C2 intermediates, and these are preferentially stabilized on different sites. Here we pursued the synthesis of catalysts in which a high-oxygen-affinity metal (such as Sn in the best catalysts herein) is present in dilute quantities within a Cu matrix. The Sn–Cu catalyst is then formed into a catalyst/carbon/ionomer heterojunction architecture that reverses electro-osmotic drag to concentrate the n-propanol produced. We achieve n-propanol electrosynthesis from carbon monoxide with a Faradaic efficiency of 47 ± 3% and a concentration of 30 wt% at an energy efficiency of 24%. We report stable n-propanol electrosynthesis for 120 h in a membrane-electrode assembly electrolyser. Electrosynthesis of n-propanol from CO has been limited by poor selectivity and low product concentration. Here a Sn–Cu catalyst/carbon/ionomer heterojunction is prepared where the adjacent atomic active sites favour the coupling of C1 and C2 intermediates to C3 product with 47% Faradaic efficiency and the reversal of electro-osmotic drag concentrates the product to 30 wt%.

用稀合金催化剂电合成正丙醇
正丙醇是一种重要的工业溶剂,但目前生产正丙醇的工业路线依赖化石燃料,二氧化碳排放量高。用可再生能源驱动的电化学系统取代化石过程,为降低正丙醇生产的碳强度提供了一条途径。通过一氧化碳电还原法电合成正丙醇依赖于C1和C2中间体的偶联,这两个中间体在不同的位点上优先稳定。在这里,我们追求高氧亲和金属(如最好的催化剂中的锡)在Cu基体中以稀量存在的催化剂的合成。然后,Sn-Cu催化剂形成催化剂/碳/离子异质结结构,逆转电渗透阻力,浓缩所产生的正丙醇。我们实现了以一氧化碳为原料的正丙醇电合成,其法拉第效率为47±3%,浓度为30 wt%,能量效率为24%。我们报告了在膜电极组装电解槽中稳定电合成120小时的正丙醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


