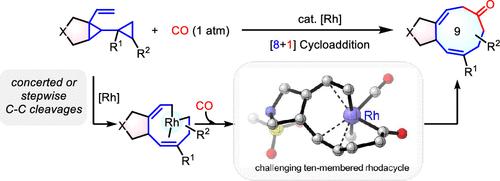
Rh-Catalyzed [8+1] Cycloaddition of Vinyl Biscyclopropanes with CO for the Synthesis of Nine-Membered Carbocycles
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Transition-metal-catalyzed cycloadditions to access nine-membered carbocycles are challenging, with only three documented examples so far. Here, we report the design of an eight-carbon synthon, vinyl biscyclopropanes (VBCPs), which undergoes Rh-catalyzed [8 + 1] cycloaddition with CO to furnish nine-membered carbocycles. This strategy enables the synthesis of 5/9 bicyclic compounds from VBCPs with diverse substituents. Mechanistic studies via quantum chemistry calculations revealed concerted C–C bond cleavages in the two cyclopropyl moieties of cis-VBCP substrates, whereas a stepwise pathway is adopted by trans-VBCPs. The key intermediate in the [8 + 1] cycloaddition is a nine-membered rhodacycle, which undergoes CO insertion followed by reductive elimination to deliver the desired nine-membered carbocycle. However, a competing β-H elimination pathway diverts the reaction, yielding a triene side product. For less effective or unsuccessful substrates, the sluggish CO insertion in the [8 + 1] cycloaddition pathway is attributed to the transannular interaction and unfavorable entropic effect during the formation of a challenging 10-membered rhodacycle in the CO insertion transition state, posing a similar obstacle for designing cycloadditions with ring sizes larger than nine.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


