Late-stage functionalization of pharmaceuticals by C–C cross-coupling enabled by wingtip-flexible N-heterocyclic carbenes
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
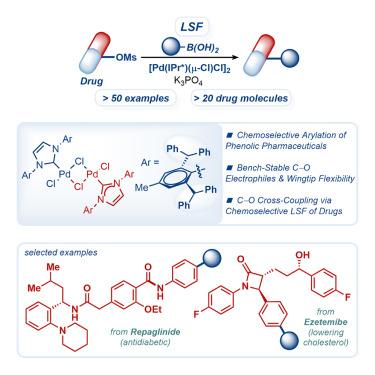
The synthesis of complex molecules by palladium-catalyzed cross-coupling has been pivotal in all stages of drug discovery research. However, this approach has been generally restricted to classical aryl halide electrophiles, requiring the use of a limited pool of precursors. Herein, we report the first highly chemoselective approach to the cross-coupling of bench-stable C–O electrophiles in which abundant phenols can be systematically used as electrophilic cross-coupling partners. Using this approach, we have achieved late-stage functionalization of >20 pharmaceuticals covering various architectures and drug targets. Wingtip-flexible N-heterocyclic carbenes as ancillary ligands enable us to address the major challenges to this mode of catalysis, such as fast oxidative addition to prevent the hydrolysis of C–O electrophiles and facile reductive elimination to establish C–C bond formation in complex settings. The design of wingtip-flexible N-heterocyclic carbene ligands will enable the cross-coupling of a broad range of electrophiles for the development of important medicines.
翼尖柔性n杂环碳烯通过C-C交叉偶联实现药物的后期功能化
钯催化交叉偶联合成复杂分子在药物发现研究的各个阶段都是至关重要的。然而,这种方法通常仅限于传统的芳卤亲电试剂,需要使用有限的前体。在此,我们报告了第一个高化学选择性的方法来交叉耦合实验稳定的C-O亲电试剂,其中丰富的酚可以系统地用作亲电交叉偶联伙伴。使用这种方法,我们已经实现了20种药物的后期功能化,涵盖了各种结构和药物靶点。Wingtip-flexible n -杂环carbenes作为辅助配体使我们能够解决这种催化模式的主要挑战,例如快速氧化加成以防止C-O亲电试剂的水解,以及在复杂环境中易于还原消除以建立C-C键形成。翼尖柔性n杂环碳配体的设计将使广泛的亲电试剂的交叉偶联成为重要药物的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


