Midkine, a novel MCP-1 activator mediated PM2.5-aggravated experimental pulmonary fibrosis
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
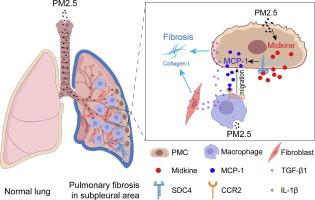
Exposure to fine particulate matter (PM2.5) is associated with increased morbidity and mortality among patients with idiopathic pulmonary fibrosis (IPF). Pathological alterations in IPF typically originate in the subpleural regions of the lungs. However, it was unclear how PM2.5 affected subpleural pulmonary fibrosis. In this study, atmospheric PM2.5 and carbon blacks were utilized as representative particulate matter to investigate these effects. Mouse models and cell models were made to investigate macrophage chemotaxis changes under PM2.5 exposure in vivo and in vitro. The findings indicated that PM2.5 promoted macrophage aggregation in the subpleural region of lung and aggravated bleomycin-induced pulmonary fibrosis in mice. At the same time, we uncovered for the first time that PM2.5 exposure led to an upregulation of midkine, which subsequently enhanced the production of monocyte chemotactic protein-1 (MCP-1) through the cell surface receptor Syndecan 4 (SDC4) in pleural mesothelial cells (PMCs), thereby, inducing macrophage aggregation in subpleural region of lung. Furthermore, our results indicated that PM2.5 and bleomycin facilitated macrophage M1 polarization and the production of profibrotic inflammatory factors, culminating in fibrotic alterations in PMCs, lung fibroblasts, and alveolar epithelial cells. Finally, we demonstrated that inhibition of midkine ameliorated lung function and mitigated pulmonary fibrosis in vivo. In conclusion, our findings elucidated that midkine acted as a novel MCP-1 activator, mediating PM2.5-aggravated experimental pulmonary fibrosis, and suggested that the midkine/SDC4/MCP-1 signal should be a new therapeutic target for the treatment of PM2.5-related IPF.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


