IL-17RA signaling provides dual tumor-suppressor function during late-stage colorectal carcinogenesis
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
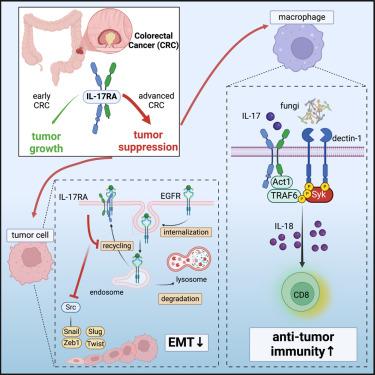
Expression of interleukin (IL)-17 family cytokines is associated with tumor-promoting inflammation. We found that low expression of IL17RA associated with worse prognosis in late-stage colorectal cancer (CRC) patients. Deletion of Il17ra in intestinal epithelial cells (IECs) in a murine model of CRC enhanced epithelial-to-mesenchymal transition (EMT) via increased expression of the epidermal growth factor receptor and subsequent activation of the kinase Src. Yet, these mice were protected from metastatic disease; Il17ra deletion impaired intestinal barrier function and enhanced systemic fungal invasion and associated immunity. However, in macrophages, IL-17RA was required for spleen tyrosine kinase (Syk) activation upon fungal-induced dectin-1 engagement, and Il17ra ablation impaired IL-18 release and protective CD8+ T cell-mediated anti-tumor immunity. Combining recombinant IL-17 and heat-killed Candida albicans rendered colorectal tumors sensitive to α-PD-1 treatment in a model of microsatellite stable (MSS) CRC. Thus, IL-17RA engages two distinct tumor-suppressive mechanisms in CRC, linking EMT and fungal-induced anti-tumor immunity during tumor progression.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


