A heart-brain-spleen axis controls cardiac remodeling to hypertensive stress
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Hypertensive heart disease (HTN-HD) meaningfully contributes to hypertension morbidity and mortality. Initially established as an adaptive response, HTN-HD progresses toward worsening of left ventricule (LV) function and heart failure (HF). Hypertensive stress elevates sympathetic nervous system (SNS) activity, a negative clinical predictor, and expands macrophages. How they interact in the compensatory phase of HTN-HD is unclear. We report that LV pressure overload recruited a brainstem neural circuit to enhance splenic SNS and induce placental growth factor (PlGF) secretion. During hypertensive stress, PlGF drove the proliferation of self-renewing cardiac resident macrophages (RMs) expressing its receptor neuropilin-1 (NRP1). Inhibition of the splenic neuroimmune axis or ablation of NRP1 in RM hindered the adaptive response to hypertensive stress, leading to HF. In humans, circulating PlGF correlated with cardiac hypertrophy, and failing hearts expressed NRP1 in RMs. Here, we discovered a multiorgan response driving a neural reflex to expand cardiac NRP1+ RM and counteract HF.
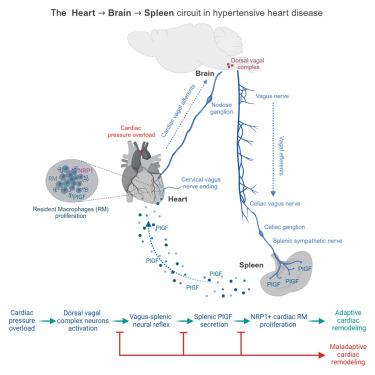
心脏-脑-脾轴控制高血压应激下的心脏重塑
高血压性心脏病(HTN-HD)是高血压发病率和死亡率的重要因素。HTN-HD最初被认为是一种适应性反应,但其进展会导致左心室(LV)功能恶化和心力衰竭(HF)。高血压应激升高交感神经系统(SNS)活性,这是一个阴性的临床预测因子,并扩大巨噬细胞。它们在HTN-HD的代偿期如何相互作用尚不清楚。我们报道左室压力过载招募了脑干神经回路来增强脾SNS并诱导胎盘生长因子(PlGF)的分泌。在高血压应激过程中,PlGF促进自我更新的心脏巨噬细胞(rm)增殖,表达其受体neuropilin-1 (NRP1)。抑制脾神经免疫轴或消融RM中的NRP1阻碍了对高血压应激的适应性反应,导致HF。在人类中,循环PlGF与心脏肥厚相关,衰竭的心脏在rmm中表达NRP1。在这里,我们发现了一种多器官反应,驱动神经反射来扩大心脏NRP1+ RM并抵消HF。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


