Integrated computational analysis identifies therapeutic targets with dual action in cancer cells and T cells
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Many cancer drugs that target cancer cell pathways also impair the immune system. We developed a computational target discovery platform to enable examination of both cancer and immune cells so as to identify pathways that restrain tumor progression and potentiate anti-tumor immunity. Immune-related CRISPR screen analyzer of functional targets (ICRAFT) integrates immune-related CRISPR screen datasets, single-cell RNA sequencing (scRNA-seq) data, and pre-treatment RNA-seq data from clinical trials, enabling a systems-level approach to therapeutic target discovery. Using ICRAFT, we identified numerous targets that enhance both cancer cell susceptibility to immune attack and T cell activation, including tumor necrosis factor (TNF) alpha-induced protein 3 (TNFAIP3), protein tyrosine phosphatase non-receptor type 2 (PTPN2), and suppressor of cytokine signaling 1 (SOCS1). In cancer cells, Tnfaip3 (A20) deletion activated the TNF-nuclear factor kappa-B (NF-κB) pathway, promoting chemokine expression and T cell recruitment to the tumor. T cell-mediated elimination of Tnaifp3-null cancer cells was primarily driven by TNF-induced apoptosis. Inactivation of Tnfaip3 in T cells enhanced anti-tumor efficacy. By integrating diverse functional genomics and clinical datasets, ICRAFT provides an interactive resource toward a deeper understanding of anti-tumor immunity and immuno-oncology drug development.
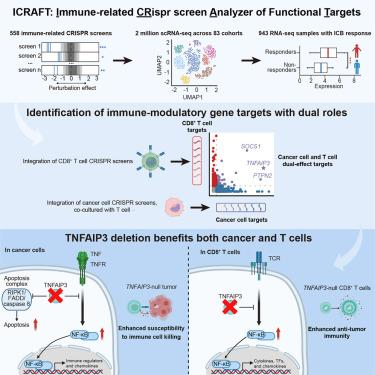
综合计算分析确定在癌细胞和T细胞中具有双重作用的治疗靶点
许多针对癌细胞途径的抗癌药物也会损害免疫系统。我们开发了一个计算靶点发现平台,可以同时检查癌症和免疫细胞,从而确定抑制肿瘤进展和增强抗肿瘤免疫的途径。免疫相关CRISPR功能靶点筛选分析仪(ICRAFT)集成了免疫相关CRISPR筛选数据集、单细胞RNA测序(scRNA-seq)数据和临床试验的预处理RNA-seq数据,使系统级方法能够发现治疗靶点。使用ICRAFT,我们发现了许多增强癌细胞对免疫攻击和T细胞活化的易感性的靶点,包括肿瘤坏死因子(TNF) α诱导的蛋白3 (TNFAIP3)、蛋白酪氨酸磷酸酶非受体2 (PTPN2)和细胞因子信号传导1 (SOCS1)的抑制因子。在癌细胞中,Tnfaip3 (A20)缺失激活了tnf -核因子κ b (NF-κB)通路,促进趋化因子表达和T细胞向肿瘤募集。T细胞介导的tnaifp3缺失癌细胞的消除主要由tnf诱导的细胞凋亡驱动。T细胞中Tnfaip3的失活增强了抗肿瘤效果。通过整合不同的功能基因组学和临床数据集,ICRAFT为更深入地了解抗肿瘤免疫和免疫肿瘤药物开发提供了一个互动资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


