Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Necrotizing enterocolitis (NEC) is a common pediatric emergency primarily afflicting preterm infants, yet its mechanisms remain to be fully understood. Here, we report that plasma fibroblast growth factor (FGF)19, a target of farnesoid X receptor (FXR), was positively correlated with the clinical parameters of NEC. NEC patients and the NEC murine model displayed abundant FXR in intestinal epithelial cells (IECs), which was restricted by microbiota-derived short-chain fatty acids (SCFAs) under homeostasis. Genetic deficiency of FXR in IECs caused remission of NEC. Mechanistically, FXR facilitated ferroptosis of IECs via targeting acyl-coenzyme A synthetase long-chain family member 4 (Acsl4). Lipid peroxides released by ferroptotic IECs suppressed interleukin (IL)-22 secretion from type 3 innate lymphoid cells (ILC3s), thereby exacerbating NEC. Intestinal FXR antagonist, ACSL4 inhibitor, and ferroptosis inhibitor ameliorated murine NEC. Furthermore, the elevated lipid peroxides in NEC patients were positively correlated with FGF19 and disease parameters. These observations demonstrate the therapeutic value of targeting intestinal FXR and ferroptosis in NEC treatment.
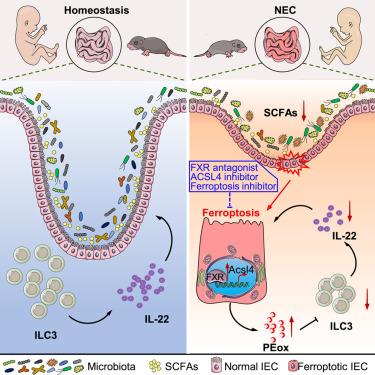
胆汁酸受体FXR促进新生儿坏死性小肠结肠炎的肠上皮铁上落和随后的ILC3功能障碍
坏死性小肠结肠炎(NEC)是一种常见的儿科急症,主要影响早产儿,但其机制仍未完全了解。在这里,我们报道了血浆成纤维细胞生长因子(FGF)19, farnesoid X受体(FXR)的靶标,与NEC的临床参数呈正相关。NEC患者和NEC小鼠模型在肠道上皮细胞(IECs)中显示出丰富的FXR,在稳态下受微生物源性短链脂肪酸(SCFAs)的限制。IECs中FXR的遗传缺陷导致NEC的缓解。机制上,FXR通过靶向酰基辅酶A合成酶长链家族成员4 (Acsl4)促进IECs铁凋亡。铁致iec释放的脂质过氧化物抑制3型先天淋巴样细胞(ILC3s)分泌白细胞介素(IL)-22,从而加重NEC。肠道FXR拮抗剂、ACSL4抑制剂和铁下垂抑制剂可改善小鼠NEC。此外,NEC患者脂质过氧化物升高与FGF19和疾病参数呈正相关。这些观察结果表明,针对肠道FXR和铁下垂在NEC治疗中的治疗价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


